Abstract
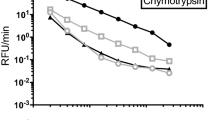
Leucine carboxypeptidase (EC 3.4.16) activity in Actinomucor elegans bran koji was investigated via absorbance at 507 nm after stained by Cd-nihydrin solution, with calibration curve A, which was made by a set of known concentration standard leucine, calibration B, which was made by three sets of known concentration standard leucine solutions with the addition of three concentrations inactive crude enzyme extract, and calibration C, which was made by three sets of known concentration standard leucine solutions with the addition of three concentrations crude enzyme extract. The results indicated that application of pure amino acid standard curve was not a suitable way to determine carboxypeptidase in complicated mixture, and it probably led to overestimated carboxypeptidase activity. It was found that addition of crude exact into pure amino acid standard curve had a significant difference from pure amino acid standard curve method (p < 0.05). There was no significant enzyme activity difference (p > 0.05) between addition of active crude exact and addition of inactive crude kind, when the proper dilute multiple was used. It was concluded that the addi-tion of crude enzyme extract to the calibration was needed to eliminate the interference of free amino acids and related compounds presented in crude enzyme extract.
Similar content being viewed by others
References
Moore, S. and Stein, W.H., J. Biol. Chem., 1948, vol. 176, pp. 367–388.
Moore, S. and Stein, W.H., J. Biol. Chem., 1954, vol. 211, pp. 907–913.
Chikuma, T., Kishii, M., Taguchi, K., Yajima, R., Kato, T., Loh, Y.P., Ishii, Y., and Tanaka, A. J. Chromatogr. B. Biomed. Sci. Appl., 1997, vol. 703, pp. 45–51.
Mao, S.S., Colussi, D., Bailey, C., Bosserman, M.M., Burlein, C., Gardell, S.J., and Carroll, S.S., Anal. Biochem., 2003, vol. 319, pp. 159–170.
Banerjee, S., Kaplan, H., Yagodnik, C., Breuil, C., and Brown, D.L., Biotechnol. Tech., 1995, vol. 9, pp. 241–246.
Heylen, E., Augustyns, K., and Hendriks, D., Anal. Biochem., 2010, vol. 403, pp. 114–116.
Hendriks, D., Scharpe, S., Sande, van M., Lommaert, M.-P., and Kasahara, Y., Anal. Biochem., 1987, vol. 164, pp. 90–95.
Logan, D.A. and Disanto, M.E., Arch. Microbiol., 1992, vol. 155, pp. 492–498.
Yemm, E.W. and Cocking, E.C., Analyst (Cambrage, U.K.), 1955, vol. 80, pp. 209–214.
Yokoyama, S. and Hramatsu, J.I., J. Biosci. Bioeng., 2003, vol. 95, pp. 204–205.
Doi, E., Shibata, D., and Matoba, T., Anal. Biochem., 1981, vol. 118, pp. 173–184.
Baer, A., Ryba, I., Meyer, J., and Butikofer, U., Lebensm. Wiss. Technol., 1996, vol. 29, pp. 58–62.
Macedo, A.C., Vieira, M., Pocas, R., and Malcata, F.X., Int. Dairy J., 2000, vol. 10, pp. 769–774.
Folkertsma, B. and Fox, P.F., J. Dairy Res., 1992, vol. 59, pp. 217–224.
Liu, F. and Yasuda, M., J. Ind. Microbiol. Biotechnol., 2005, vol. 32, pp. 487–489.
Arai, S., Yamashita, M., Kato, H., and Fujimaki, M., J. Food Sci., 1970, vol. 35, pp. 392–395.
Komai, T., Kawabata, C., Tojo, H., Gocho, S., and Ichishima, E., Fisheries Sci., 2007, vol. 73, pp. 404–411.
Umetsu, H., Matsuoka, H., and Ichishima, E., J. Agric. Food Chem., 1983, no. 31, pp. 50–53.
Li, L., Yang, Z.Y., Yang, X.Q., Zhang, G.H., Tang, S.Z., and Chen, F., J. Ind. Microbiol. Biotechnol., 2008, vol. 35, pp. 41–47.
Ozcan, M. and Akman, S., Spectrochimica Acta Part B: Atom. Spectrosc., 2005, vol. 60, pp. 399–402.
Zhu, Y.H., Li, G.R., Duan, Y.P., Chen, S.Q., Zhang, C., and Li, Y.F., Food Chem., 2008, vol. 109, pp. 899–908.
Ito, S. and Tsukada, K., J. Chromatogr. A, 2002, vol. 943, pp. 39–46.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Fu, J., Li, L., Yang, X.Q. et al. Application of standard addition for the determination of carboxypeptidase activity in Actinomucor elegans bran koji. Appl Biochem Microbiol 47, 556–562 (2011). https://doi.org/10.1134/S000368381105005X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000368381105005X