Abstract
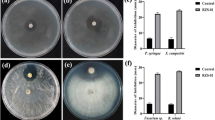
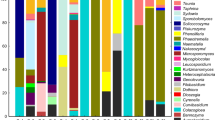
In this study, a total of 1125 actinobacteria were isolated from the selected mangrove species: Avicennia marina, Rhizopora mucronata and Ceriops tagal from three study stations viz., Minnie Bay, Carbyn’s Cove and Burmanallah. Among these three stations, the highest number of actinobacteria was recorded in Carbyn’s Cove (64.97%), followed by (25.51%) at Burmanallah and the minimum of (9.51%) was recorded in Minnie Bay. Maximum number of actinobacteria was recorded from Ceriops tagal (40.44%) than the other selected mangrove species Avicennia marina (34.13%) and Rhizopora mucronata (25.42%). Among the 1,125 mangrove-associated actinobacteria, 103 morphologically different isolates from the Minnie Bay station was selected for the further characterization studies. In antibacterial assay, 30.11% of the isolates revealed inhibitory activity against all tested clinical pathogens and 65% isolates displayed inhibitory activity against minimum of 04 tested clinical pathogens. Growth survival studies of the actinobacterial isolates also accomplished to withstand in varied NaCl and pH levels. Of 103 isolates, all were found to synthesize gelatinase enzyme, 73 isolates demonstrated amylolytic activity, 38 isolates exhibited proteolytic and 63 isolates displayed urease activity. Interestingly, 56 isolates exhibited excellent DNase activity and 71 isolates revealed positive for l-asparaginase production. To our recognition, 11 isolates exhibited constructive results in the production of 06 extracellular enzymes of industrial importance. Of 103 isolates, 48 isolates were confirmed by molecular level identification. Based on the phylogenetic analysis, the isolates were categorized under the genera: Streptomyces, Nocardiopsis, Salinispora and Actinomadura.










Similar content being viewed by others
Availability of data and materials
Not Applicable.
References
Kathiresan NK, Bingham BL. Biology of mangroves and mangrove ecosystems. Adv Mar Biol. 2001;40:81–251.
Brown BE. Integrated coastal management: South Asia. Newcastle, Newcastle upon Tyne: Department of Marine Sciences and Coastal Management, Univ; 1997.
Baskaran R, Vijayakumar R, Mohan PM. Enrichment method for the isolation of bioactive actinomycetes from mangrove sediments of Andaman islands, India. Malays J Microbiol. 2011;7:1–7.
Bredholt H, Fjaervik E, Jhonsen G, Zotechev SB. Actinomycetes from sediments in the Trondhein Fjrod, Norway: diversity and biological activity. Mar Drugs. 2008;6:12–24.
Pelaez F. The historical delivery of antibiotic from microbial natural products—can history repeat? Biochem Pharmacol. 2006;71:981–90.
Veiga M, Esparis A, Fabregas J. Isolation of cellulolytic actinomycetes from marine sediments. Appl Environ Microbiol. 1983;46:286–7.
Jensen PR, Gontang E, Mafnas C, Mincer TJ, Fenical W. Culturable marine actinomycetes diversity from tropical Pacific ocean sediments. Appl Environ Microbiol. 2005;7:1039–48.
Kumar KS, Sahu MK, Kathiresan K. Isolation and characterization of streptomycetes producing antibiotic, from a mangrove environment. Asian J Microbiol Biotechnol Environ Exp Sci. 2005;7:457–64.
Jeffrey LSH, SahilahSon AMR, Tosiah S. Isolation and screening of actinomycetes from Malaysian soil for their enzymatic and antimicrobial activities. J Tropical Agric Food Sci. 2007;35:159–64.
Oskay AM, Usame T, Cem A. Antibacterial activity of some actinomycetes isolated from farming soils of Turkey. African J Biotechol. 2004;3:441–6.
Meena B, Anburajan L, Vinithkumar NV, Kirubagaran R. Novel marine actinobacteria from emerald Andaman & Nicobar islands: a prospective source for industrial and pharmaceutical byproducts. BMC Microbiol. 2013;13:145.
Ellaiah P, Kalyan D, Rao VS, Rao BV. Isolation and characterization of bioactive actinomycetes from marine sediments. Hindustan Antibiot Bull. 1996;38:48–52.
Kuster E, Williams S. Selection of media for the isolation of Streptomyces. Nature. 1964;202:928–9.
Weyland H. Actinomycetes in North sea and Atlantic ocean sediments. Nature. 1969;223:858.
Shirling EB, Gottileb D. Methods for characterization of Streptomyces species. Int J Syst Bactriol. 1966;16:312–40.
Pridham TG, Gottlieb D. The utilization of carbon compounds by some actinomycetes as an aid for species determination. J Bacteriol. 1948;56:107–14.
Williams ST, Cross T. Actinomycetes, methods in microbiology, vol. 4. New York: Academic Press; 1971.
Lemos ML, Toranzo AE, Barja JL. Antibiotic activity of epiphytic bacteria isolated from intertidal seaweeds. Microb Ecol. 1985;11:149–63.
Salle AJ. Laboratory manual on fundamental principles of bacteriology. UK: McGraw Hill; 1948.
Leon J, Liza L, Soto I, Cuadra D, Patino L, Zerpa R. Bioactive actinomycetes of marine sediment from the central coast of Peru. Rev Peru Biol. 2007;14:259–70.
Gordon RE, Mihm JM. A comparative study of some strains received as Nocardiae. J Bacteriol. 1957;73:15–27.
Gulati R, Saxena RK, Gupta R. A rapid plate assay for screening L-asparaginase producing microorganisms. Lett Appl Microbiol. 1997;24:23–6.
Kutchama AJ, Roberts MA, Knaebel DB, Crawford DL. Small-scale isolation of genomic DNA from Streptomyces mycelia or spores. Biotechniques. 1998;24:452–6.
Altschul SF, Thomas LM, Alejandro AS, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9.
Talbot GH, Bradley J, Edwards JE Jr, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the infectious diseases society of America. Clin Infect Dis. 2006;42:657–68.
Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–9.
Vanajakumar G, Selvakumar N, Natarajan R. Antagonistic properties of actinomycetes isolated from mollusks of the porto Novo region. South India. 1995: 267–274.
Saurav K, Kannabiran K. Diversity and optimization of process parameters for the growth of Streptomyces VITSVK9 sp. isolated from Bay of Bengal, India. J Nat Environ Sci. 2010;1:56–65.
Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thompson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature. 2002;417:141–7.
Hong K, Gao AH, Xie QY, Gao H, Zhuang L, Lin HP, Yu HP, Li J, Yao XS, Goodfellow M, Ruan JS. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar Drugs. 2009;7:24–44.
Pandey A, Shukla A, Majumdar SK. Utilization of carbon and nitrogen sources by Streptomyces kanamyceticus M27 for the production of an anti-bacterial antibiotic. Afr J Biotech. 2005;4:909–10.
Ramesh S, Rajesh M, Mathivanan N. Characterization of a thermostable alkaline protease produced by marine Streptomyces fungicidicus MML1614. Bioprocess Biosyst Eng. 2009;32:791–800.
Singh SP, Thumar JT, Gohel SD and Purohit MK. Molecular diversity and enzymatic potential of salt-tolerent alkaliphilic actinomycetes. In Mendez A, editors. Current research, technology and education topics in applied microbiology and microbial biotechnology; 2010.
Luo HY, Wang YR, Miao LH, Yang PL, Shi PJ, Fang CX, Yao B, Fan YL. Nesterenkonia alba sp. nov., an alkaliphilic actinobacterium isolated from the black liquor treatment system of a cotton pulp mill. Int J Syst Evol Microbiol. 2009;59:863–8.
Vasavada SH, Thumar JT, Singh SP. Secretion of a potent antibiotic by salt- tolerent and alkaliphilic actinomycete Streptomyces sannanensis strain RJT-1. Curr Sci. 2006;91:1393–7.
Okami Y. Marine microorganisms as a source of bioactive agents. Microb Ecol. 1986;12:65–78.
Bernfield P. Amylases α and β. In: Methods in enzymology. 1st edition. New York, USA: Academic Press; 1955. p. 149–58.
Acknowledgements
The author’s great fully acknowledge the financial support given by the Ministry of Earth Sciences, Government of India, New Delhi, to conduct the survey and research. The authors are thankful to Dr. M. A. Atmanand, Director, National Institute of Ocean Technology, Chennai for providing necessary facilities to perform this research.
Funding
Not Applicable.
Author information
Authors and Affiliations
Contributions
The research concept and the experiments were executed by BM, LA and MAJ. NVV and GD analyzed the data and reviewed the manuscript. All of the authors approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meena, B., Anburajan, L., Johnthini, M.A. et al. Exploration of mangrove-associated actinobacteria from South Andaman Islands, India. Syst Microbiol and Biomanuf 3, 702–718 (2023). https://doi.org/10.1007/s43393-022-00134-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-022-00134-3