Abstract
In the European Industry, 275 TWh of thermal energy is rejected into the environment at temperatures beyond 300 °C. To recover some of this wasted energy, bottoming thermodynamic cycles using supercritical carbon dioxide (sCO2) as working fluid are a promising technology for the conversion of the waste heat into power. CO2 is a non-flammable and thermally stable compound, and due to its favourable thermo-physical properties in the supercritical state, can lead to high cycle efficiencies and a substantial reduction in size compared to alternative heat to power conversion technologies. In this work, a brief overview of the sCO2 power cycle technology is presented. The main concepts behind this technology are highlighted, including key technological challenges with the major components such as turbomachinery and heat exchangers. The discussion focuses on heat to power conversion applications and benefits of the experience gained from the design and construction of a 50 kWe sCO2 test facility at Brunel University London. A comparison between sCO2 power cycles and conventional heat to power conversion systems is also provided. In particular, the operating ranges of sCO2 and other heat to power systems are reported as a function of the waste heat source temperature and available thermal power. The resulting map provides insights for the preliminary selection of the most suitable heat to power conversion technology for a given industrial waste heat stream.
Similar content being viewed by others
1 Introduction
The more stringent national and international regulations on greenhouse gas emissions as well as the increasing environmental concerns are driving academia and industry to seek new sustainable solutions to meet the growing energy demand. Apart from enhancing a higher penetration of renewables in the energy mix, the energy efficiency improvement of existing industrial and power generation facilities is considered as essential to achieve the aforementioned targets.
One of the approaches to lower the carbon footprint of the current industrial sector is represented by the recovery and re-utilization of the heat lost after combustion and heat transfer processes, whose relevance has been estimated in the 63% of the global primary energy consumption in industry [1]. This amount of waste thermal energy includes the heat rejected into the environment as effluents and exhausts. On the other hand, the thermal energy dissipated in irreversible processes such as friction losses, electrical resistance and transmission is unlikely to be recoverable.
The exploitation of potential is hence crucial and, depending on the characteristics of the waste heat source, it involves technical challenges. For instance, different technologies must be considered if the waste heat source is available at low (< 100 °C), medium (100–300 °C) or high temperature levels (> 300 °C) [2]. Other factors to take into account are the temporal availability of the waste source, the composition of the heat carrier, the intensity or modality of supply but also the economic and financial feasibility of the retrofit and the utilization of the recovered energy [2].
In particular, two main approaches can be pursued, the direct re-use of the waste heat recovered or its conversion into electric power. Unlike the direct use of the recovered heat that requires a heat demand in the industrial site or in the nearby ones, an electrical energy recovery is more favorable in terms of energy management, since the surplus of electricity can be dumped to the electrical grid [3]. In addition, electricity is considered more valuable from an economic perspective [2].
The conversion of waste heat into electrical power has been conventionally addressed in the last decades through power units based on steam or Organic Rankine cycles (ORC). These technologies are similar in the underpinning thermodynamics but present significant technical and economic differences at applied level. In general terms, ORC units are more suitable at small-scale, i.e. tens or hundreds of electrical kilowatts, while steam power cycles are more suitable at megawatt scale [4]. Both technologies however show some shortcomings and limitations in terms of temperature and efficiency which are addressed in this paper. Supercritical carbon dioxide (sCO2) power cycles represent a promising solution thanks to their compactness, high efficiency and operational flexibility [5].
Supercritical CO2 power systems have been investigated and reviewed for nuclear, concentrated solar and advanced power generation applications [6,7,8]. In this work, instead, an overview on sCO2 power technology is presented, with a particular focus on the potential use of these systems for waste heat to power conversion applications. Firstly, a map comparing the different operating ranges of the available heat to power conversion technologies as a function of the main parameters identifying the waste heat source is showed in Sect. 2. In this analysis, the operating range of sCO2 power technology is specified, showing that these systems can represent a breakthrough for the sector. Afterwards, the main concept behind the technology is presented in Sect. 3 and an up to date review of progresses as well as the technical challenges in the main component’s development is detailed in Sect. 4. This review includes also novel information on the design and selection of heat exchangers and materials acquired by the authors during the construction of a 50 kW sCO2 testing facility for waste heat recovery (WHR) applications at Brunel University London [9].
2 Heat to power conversion applications
Originally studied for the nuclear sector because of the lower chemical reactivity of CO2 with molten sodium [10] (most promising candidate as primary heat carrier in next generation nuclear reactors), sCO2 systems have been also investigated for fossil fueled power plants, because of their high operational flexibility and ease of implementation of carbon capture and storage systems [11,12,13].
Supercritical CO2 power cycles are also being considered in the renewable energy sector, particularly for Concentrated Solar Power (CSP) and geothermal applications. In the latter case, the use of CO2 allows the exploitation of geothermal sources at a higher depth and temperature levels, increasing the maximum efficiency and power output obtainable with more conventional technologies (as for instance Organic Rankine Cycle systems) [14]. In the CSP sector, the benefits are instead more related to the consistent reduction of the production cost of electricity thanks to the possibility of directly recovering the solar radiation without using any intermediate energy carrier, although this poses challenges in the design of high pressure solar collectors and receivers, as well as energy storage systems [7, 15, 16].
2.1 High-grade waste heat potential
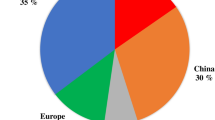
Equally promising can be the use of the technology for waste heat recovery (WHR) applications. As showed by Fig. 1, the potential energy recovery only in the European industrial sector, which accounts of the 25.9% of the primary energy consumptions (Fig. 1a), has been estimated to be around 918 TWh (Fig. 1c). This potential energy waste is rejected into the environment through exhausts and effluents and it is widely distributed across the main European sites, being supplied at low (< 100 °C), medium (100–270 °C), and high (> 270 °C) temperature levels.
Theoretical and Carnot’s waste heat recovery potentials in EU28 industrial sector: overall EU28 energy consumptions (a), overall industrial consumptions (b), theoretical waste heat recovery potential divided by temperature levels (c) and Carnot’s WHR potential (d) [3]
As it is shown in Fig. 1c, the potential heat recovery from the low temperature waste heat sources represents the higher share (51%), However if the Carnot efficiency is considered, the high temperature waste heat sources give the higher contribution, which is equal to almost 154 TWh (55% of the total WHR potential as showed in Fig. 1d). The lack of available technologies for recovery and power conversion of high temperature waste heat makes sCO2 power cycles a promising candidate to harvest this energy waste at a competitive levelized cost of electricity.
2.2 Benchmark of supercritical CO2 heat to power cycles
Figure 2 displays the operating ranges of conventional and innovative waste heat to power conversion technologies as a function of the heat source temperature level and capacity, i.e. including type and mass flow rate of the waste exhaust or effluent. Although Organic Rankine Cycle (ORC) systems proved to be a successful technical solution especially for large scale applications [17], the use of this technology is limited in a range of temperatures of the waste heat source that goes from 100 °C up to 400 °C [18]. The upper limit is imposed by the flammability and low chemical stability of the organic fluids at high temperatures, while the lower one by their vapour pressure which, in turn, limits the efficiency and the output of ORC units at extremely low temperatures.
For waste source capacities from 10 kW up to 200 kW, ORC systems equipped with positive displacement machines are preferred to the ones with axial or centrifugal turbines [19] (Fig. 2). In fact, in this power range, volumetric machines can achieve higher efficiencies compared to dynamic ones, whose reduced size leads to losses. Furthermore, positive displacement machines benefit from a reduced installation and maintenance complexity due to lower revolution speeds, reduced vibration levels and wider range of optimal operating conditions [20].
For power scales between 200 kW and 15 MW (the larger ORC installation to date [21]), turbines are instead adopted since their size can be increased with consequent benefits in efficiency [22].
Steam Rankine cycles are usually preferred to ORC to exploit waste heat sources with higher thermal capacities (from 10 MW up to hundreds of MW) [23], because of the higher efficiency and the lower capital cost due to more standard components [23]. The operating range of this technology can be further extended to power scales lower than 10 MW using micro steam turbines (Fig. 2) which are however characterised by lower performance than large machines due to high tip leakage losses [23].
The temperature range at which the Steam Rankine technology is usually employed goes from 250 °C up to 700 °C (Fig. 2) [23, 24]. The lower limit is given by the low vapour pressure of water, while the upper one from material and technological constraints. More advanced units, as the ultra-supercritical steam power systems, can also exploit heat sources beyond 620 °C, but they require significant additional investment costs [23].
Waste heat source available at temperature levels lower than 100 °C can be still exploited by adopting the Trilateral Flash Cycle (TFC) technology (Fig. 2). In these kind of systems, the organic working fluid is heateduntil the saturated liquid conditions) and undergoes to a two-phase expansion [25]. For these reasons, these units are suitable for ultra-low temperature WHR applications, from 200 °C down to 70 °C
Furthermore, because of the two-phase expansion, volumetric machines are usually adopted since they guarantee a higher adiabatic efficiency. The size of these machines, however, limits the maximum thermal capacity of the waste heat source exploitable, which can go up to 5 MW [26, 27]. For capacities lower than 1 MW, ORC systems are more competitive [26].
Hence, it is possible to notice that sCO2 systems fill an important gap in industrial WHR applications (Fig. 2). For waste heat source temperatures higher than 700 °C, sCO2 power cycles are the only option available and can thus constitute a breakthrough in the sector (Fig. 2). The high chemical stability of CO2 allows to directly recover and convert heat at temperatures up to 850 °C (Fig. 2), which is a limit posed by current materials [28]. The lower limit is instead set at 350 °C considering a simple regenerated layout, which represents the most convenient option for WHR applications [29]. For such low cost systems, characterized by a low cycle pressure ratio, the achievement of higher temperatures at the turbine inlet to obtain a positive net electric output is required [29].
From a power scale perspective, several technological challenges and the high investment costs set the lowest feasible unit capacity at 50 kWe [9], which correspond to a waste source thermal power of 300 kW assuming a 20% system thermal efficiency [29]. Among the technical limitations, the main ones arise from the reduced size of the turbomachines. Typical wheels diameters range from 30 mm to 50 mm, with consequent issues of leakage, high vibrations level and friction due to the elevated revolution speeds (over 60,000 RPM) [9, 30]. On the other side, it is possible to scale up sCO2 systems until tens of MW (Fig. 2), as it has been proved by the SunShot program and Echogen units [31, 32]. In this case, rather than on the turbomachinery side, which can benefit from the knowledge acquired in the gas turbine and steam power plants sectors, technological limitations arise on the scaling up of heat exchangers.
3 sCO2 power cycles
Supercritical CO2 power cycles use carbon dioxide in the supercritical phase as working fluid to convert the heat received by a given thermal source into electricity. The thermodynamic cycle usually performed is the Joule–Brayton one [33], even if some references to the Rankine cycle can be found in the literature when the condensation of the CO2 takes place during the heat rejection phase [34, 35].
The simplest configuration of a sCO2 power cycle is the simple regenerated layout presented in Fig. 3. This system is composed of three heat exchangers and two turbomachines, namely compressor and turbine. The low, medium and high temperature heat exchangers pursue different functions and, in turn, they are commonly referred to as gas cooler, recuperator and primary heater respectively. With reference to the temperature-entropy (T–s) diagram of Fig. 3b, the CO2 is pressurized in the compressor (1–2) and heated up first in the cold side of the recuperator (2–3) and then in the primary heater (3–4), where the actual heat recovery from the industrial topping process takes place (red line). Afterwards, the high enthalpy CO2 flow is expanded in the turbine (line 4–5) until a pressure close to the critical one. The CO2 temperature at the turbine outlet is further cooler down at the hot side recuperator (5–6). Finally, the initial cycle thermodynamic conditions are restored by rejecting the residual heat to a cooling source through the gas cooler (6–1).
The main advantages of this technology come from the particular properties that CO2 assumes after the critical transition. In the supercritical state, the CO2 presents a higher density which enables to downsize the equipment with respect to more conventional technologies as gas turbines or steam power plants [36]. The reduced size of the system components allows to reduce the investment and maintenance costs as well as the footprint of the system itself, which can be beneficial for WHR applications [37, 38]. A further benefit derives from the fluid properties near the critical point, which occurs at a temperature and pressure of 31.1 °C and 73.9 bar respectively. At these thermodynamic conditions, the carbon dioxide experiences high values for specific heat at constant pressure and isothermal compressibility [33]. These properties, together with a liquid-like density, ensure a reduction of the mechanical work required to pressurize the fluid, with consequent advantages in terms of cycle efficiency and power output.
The beneficial effect of a mechanical compression near the critical point is displayed in Fig. 4 through the CO2 pressure versus specific volume diagram. Assuming an ideal isothermal compression of a unitary mass of CO2 between two given pressure levels (from 75 bar to 160 bar), the green area refers to the work needed when the temperature of the fluid at the compressor inlet is equal to the critical one (31.5 °C), while the red one indicates the additional work required when the temperature is increased up to 41.5 °C.
On the other hand, the closer fluid is to the critical point and the more its thermo-physical properties change with temperature and pressure. As an example, Fig. 5 reports the variation at different pressure levels of the of the CO2 specific heat at constant pressure as a function of temperature. This poses a challenge in terms of system regulation, since accurate instrumentation is required to control the compressor inlet temperature.
The CO2 critical point also limits the maximum pressure ratio achievable in the cycle. In fact, since the minimum pressure of the cycle is fixed by the critical transition (75 bar) and the maximum one by technological constraints (typically around 250–300 bar), only a maximum pressure ratio of 4 can be achieved (which is extremely low compared to the value of 200 in Rankine steam technology [39]). To overcome these limitations, research is being carried out to develop new materials for harsh operating conditions [40] and in the field of CO2 doping, which consists in the mixing of carbon dioxide with compounds able to lower the CO2 critical pressure [41, 42].
The low cycle pressure ratio achieved leads to elevated temperatures at the end of the expansion and then a high level of recuperation is needed to obtain reasonable performance [43]. Even though this fact guarantees high thermal efficiency, at the same time it limits the system net power output, and hence high temperatures at the turbine inlet are needed to generate electric power (especially considering the slow divergence of the CO2 isobaric lines). However, at these high temperature levels, the CO2 assumes a strong corrosive behaviour, requiring the development of innovative materials to withstand at such harsh operating conditions.
4 Progress in sCO2 power cycle technology
The high potential behind the sCO2 technology has led to extensive academic and industrial research activities in the last decade. Despite these efforts, there are still a number of technological challenges that need to be addressed in order to advance the technology readiness level of such systems. The main ones relates to the development of turbomachines and heat exchangers (especially if considering very large or small power scales), as well as materials and manufacturing techniques. In this work, a particular focus has been given on heat exchangers and materials, which are seen as the key bottlenecks to the improvement of the sCO2 power cycles performance and economic feasibility. In fact, literature studies estimated that nearly 80% of the investment costs in a sCO2 power plant relates to heat transfer equipment [44].
4.1 Turbomachinery
The higher density of CO2 allows to downsize turbines and compressors and thus to decrease the overall capital and operating expenditures of the system. However, it also poses additional technological challenges in terms of machine design [45, 46], single or multiple shaft configurations [47], bearings [44, 48,49,50,51], seals [52,53,54,55], rotor dynamics and pressure containment [44]. Many of these issues arise when small-scale systems are considered and leakage problems become relevant. For larger sizes, technical solutions are available from other technological fields such as gas turbines or ultra-supercritical steam power plants.
While this holds especially for turbines, different considerations must be made for compressors. Despite the high benefits achievable in terms of efficiency and system power output, operating the compressor close to the CO2 critical point may cause the partial condensation of the working fluid at the inlet of the impeller [56, 57], with the consequent challenges deriving from a wet compression.
To overcome this limitation Poerner et al. in [58] proposed to adopt the gas ejection technology, widely diffused in the oil and gas wet compressors. A series of holes are placed at the blade inlet of the compressor impeller and used to eject a dry gas to break the liquid film formed on the airfoil. The result is the restored aerodynamic performance of the compressor and an improved lifetime of the component [58].
A further challenge derives from the management and control of the compressor. Since close to the critical point a slight change of pressure and temperature translates in a dramatic variation of the fluid thermo-physical properties (i.e. density, thermal conductivity or thermal capacity), very accurate sensors are needed to be able to regulate effectively the device and thus the overall system [59]. The characteristics of the first prototypes of turbines, compressors and Turbine-Alternator-Compressor (TAC) units designed by the different industrial and academic institutions worldwide are summarized in Tables 1 and 2 respectively.
4.2 Heat exchangers
Heat transfer equipment for sCO2 applications is fundamental to enhance the efficiency and the economic viability of this technology. While a simple regenerated sCO2 system one would require only three heat exchangers, up to five devices would be needed for more complex and performant cycle architectures [10]. These devices also represent the largest components in the system and must withstand high temperatures and pressures.
Furthermore, to avoid the excessive erosion of the already limited cycle pressure ratio, sCO2 heat exchangers must be designed to minimize the pressure drops, which is not trivial especially considering that a trade-off exists between the minimization of pressure drops, maximization of the heat duty and reduction of costs [68]. Reducing the pressure drops across heat exchangers leads to a reduction of their wet surface, which limits their effectiveness and heat transfer capabilities. Increasing the heat duty however, allows to increase cycle efficiency and power output, but requires also greater heat transfer area, thus increasing pressure drops and costs.
Additional design specifics must also be fulfilled depending on the type of heat exchanger considered. For the gas cooler, despite the lower operating pressure and temperatures, the heat duty requirements and the type of heat sink considered (air or water) can considerably affect the component design and optimization [69]. The recuperators are instead more challenging because of the higher pressures and temperatures involved. In this case also long term creep and fatigue resistance are required because of the wide range of temperatures occurring across the different heat exchanger sections and the limited operation maintenance due to the extreme compactness of these devices [44]. Even more ambitious is the design of the primary heater, which not only is the component exposed to the highest temperature and pressure of the cycle, but depending on the nature of the heat source, it may have to operate also in an extremely corrosive environment (i.e. nuclear or WHR applications). A further requirement could be the minimization of the pressure drops on both the working fluid and the heat source side, especially if exhausts are considered as heat source. Hence, considering all these aspects different technologies have been considered and investigated for sCO2 heat exchangers.
4.2.1 Primary heater technologies
Due to the aforementioned design and operational requirements, when the heat source is in a gaseous form, shell and tube (S&T) heat exchangers are usually adopted a primary CO2 heaters. The sCO2 usually flows inside the tubes, while the heat source/sink flows along the shell. Plate baffles are embedded to enhance the heat transfer, but they also lead to increased pressure drops. The main drawbacks of this heat technology are the low compactness and the high heat transfer surface required to achieve an effectiveness at least higher of 0.85 for sCO2 power cycle applications [70].
To increase the compactness of these heat exchangers and further enhance the heat transfer performance, the tube size can be reduced (tube diameter lower than 1 mm), obtaining a so-called micro-tube heat exchanger. The enhanced heat transfer coefficient obtainable thanks to the smaller tubes allows to avoid baffles and thus to decrease the pressure drops on the flue gas side, even if this leads to an increase of the pressure drops and a reduced flow velocity on the CO2 side. Further advantages of micro-tube heat exchangers with respect to the conventional shell and tube ones are a greater scalability and modularity as well as a better resistance to harsh operating conditions; on the other hand, a drawback is represented by the higher manufacturing cost due to the special welding operations required to assemble the tubes in the headers [70]. Should the heat source be an effluent, Printed Circuit Heat Exchangers (PCHEs) also become a viable option.
4.2.2 Recuperator technologies
PCHEs and Formed Plate Heat Exchangers (FPHEs) are always preferred for sCO2 recuperators, thanks to their extremely high heat duty per unit volume, compactness, creep and fatigue resistance as well their capability to withstand high pressure and temperatures. The reduced material shrinkage due to the additive manufacturing techniques can make FPHE cheaper than PCHE [71]. On the other hand, PCHE can be operated at higher pressures (up to 1000 bar) compared to the 250 bar achievable by the FPHE technology [72].
To further reduce the cost of these components, industrial and academic organizations are investigating new technical solutions. Among the different ongoing alternatives, the most promising heat exchanger technologies are the Plate-Matrix and Wire-Mesh Heat Exchangers (PMHE and WMHE) [72, 73], which guarantee even higher compactness, heat duty and lower costs due to a lower material use.
4.2.3 Gas coolers technologies
The shell and tube technology can be adopted as gas coolers if air is used as cooling medium [43, 70]. If a liquid coolant is considered, PCHE provide a compact yet pricey technological solution. At small power scales or when the footprint is not a major issue for the sCO2 system, components available from the CO2 refrigeration sector, such as Plate Heat Exchangers (PHE), can be used instead of PCHE with an extreme reduction of capital and operational costs [9]. Table 3 summarizes the heat exchanger technologies available for each of the heat exchanger typology in sCO2 power cycles as well as the average cost per kW/K of the devices.
4.3 Materials
SCO2 systems performance strongly depend on the maximum temperature achieved in the cycle. Hence, the development of suitable materials is crucial for the establishment of the technology. The challenge is even emphasized by the high cycle pressures and the strong corrosive behavior that CO2 shows at temperatures higher than 500 °C. Among the several forms of corrosion, the main relevant mechanisms that can occur in a CO2 environment are high temperature oxidation, carburization and metal dusting [76].
While high temperature oxidation is common also for more conventional working fluids (i.e. steam or air), carburization and metal dusting assume a particular relevance for CO2 applications. Both these corrosion mechanisms combined with the high temperature oxidation phenomena can lead to catastrophic failure of the system components [77]. In particular, the carburization reaction leads to the depletion of ferrous ions present in the alloys which, reacting with CO2, form carbon atoms [78]. The carbon atoms increase the rate of interstitial carbides formation along the grain boundaries of the alloy, reducing its strength and creep resistance.
The formation of these interstitial carbides also leads to the depletion of all that elements which prevent the oxidation of the alloy (i.e. chromium), so accentuating the high oxidation temperature corrosion. When metal dusting occurs, i.e. a more widespread carburization on the alloy surface, the size of the interstitial carbides increases exponentially until the carbide zone becomes super-saturated and graphite nucleation takes place. As this nucleation begins, the graphite nuclei starts to grow and break the oxide layer previously formed [76].
To overcome these issues, usually nickel, vanadium and titanium are used as alloying elements. Nickel, thanks to its poor solubility with carbon, offers good resistance to carburization since it mitigates the carbon migration towards the alloy surface. Vanadium and Titanium, on the contrary, rapidly react with carbon because of their strong chemical affinity with the compound and prevent the formation of chromium carbides. For these reasons, for the components of the sCO2 system operating at high temperature (i.e. turbine and primary heater) the most suitable material candidates are nickel rich alloys [79, 80], while stainless steel can be used for components withstanding lower temperatures (i.e. recuperators operating below 500 °C) [40].
For components subjected to less harsh operating conditions (i.e. compressor and gas cooler) aluminum alloys could also be adopted [31]. Table 4 recalls the main alloys investigated and tested for sCO2 applications classified by to their maximum operating temperature and the most suitable component.
4.4 Integral testing facilities
To prove the concept of sCO2 power cycles, different industrial and academic research institutes developed prototypes of sCO2 heat to power conversion systems. Sandia National Laboratories (SNL) and the Knolls Atomic Power Laboratories (KAPL) were the first research centers to develop two integral sCO2 power cycle test facilities for nuclear applications. Afterwards, many institutes started their own research activities, both in the United States (e.g. Argonne National Laboratories, ANL, South West Research Institute, SWRI, Bechtel Marine Propulsion Laboratories, BMPL) and in Asia, especially in Japan (Tokyo Institute of Technology, TIT) and South Korea (Korea Advanced Institute of Science and Technology, KAIST, and Korea Institute of Energy Research, KIER). In the latest years, European institutions also developed laboratory scale facilities. Table 5 summarizes the experimental test rigs currently commissioned and their main characteristics.
5 Conclusions and future research
In this work, a review of sCO2 power cycle technology has been presented. The use of CO2 as working fluid in heat to power conversion systems can provide several benefits in terms of efficiency, compactness and operational flexibility compared with other more conventional technologies. Despite the extensive research carried out in the field, the technology readiness level is still quite low.
Additional research and development is required for the scaling up of compact high temperature and pressure heat exchangers at reasonable cost, to reduce leakage and mechanical losses in turbomachines, and to ensure optimal regulation and safe operating conditions of the compressor close to the CO2 critical point. Additional research is also needed to improve the properties and reduce the cost of materials for operation at temperatures beyond 650 °C in order to enhance the overall system performance and operating lifetime.
Research programs are being carried out to evaluate the performance of sCO2 power cycles in different applications such as geothermal, concentrated solar power and nuclear power applications. Another promising application is high temperature waste heat recovery to power conversion, in particular for waste heat temperatures above 400 °C and thermal capacities in the range between 300 kW and 30 MW. At high waste heat temperatures, Organic Rankine Cycle systems have limitations due to the poor thermal stability of organic fluids and steam Rankine technology has lower efficiencies than sCO2 cycles. Further research can extend both the range of operation and power output of sCO2 systems.
Abbreviations
- BMPC:
-
Bechtel Marine Propulsion Corporation
- BUL:
-
Brunel University London
- CVR:
-
Research Centre Rez
- ENO:
-
Enogia
- FPHE:
-
Formed Plate Heat Exchanger
- GE:
-
General electric
- HT:
-
High temperature
- IAE:
-
Institute for the Atomic Energy
- KAERI:
-
Korean Atomic Energy Research Institute
- KAPL:
-
Knolls Atomic Power Laboratories
- KIER:
-
Korean Institute for Energy Research
- LT:
-
Low temperature
- MT:
-
Medium temperature
- ORC:
-
Organic Rankine Cycle
- PCHE:
-
Printed Circuit Heat Exchanger
- PHE:
-
Plate Heat Exchanger
- PMHE:
-
Plate Mesh Heat Exchanger
- sCO2 :
-
Supercritical carbon dioxide
- sCO2 HeRo:
-
Supercritical CO2 heat removal system
- SNL:
-
Sandia National Laboratories
- SWRI:
-
South West Research Institute
- TAC:
-
Turbine Alternator Compressor
- TFC:
-
Trilateral Flash Cycle
- TIT:
-
Tokyo Institute of Technology
- WHR:
-
Waste heat recovery
- WMHE:
-
Wire-mesh heat exchanger
References
Cullen JM, Allwood JM (2010) Theoretical efficiency limits for energy conversion devices. Energy 35:2059–2069. https://doi.org/10.1016/J.ENERGY.2010.01.024
Forman C, Muritala IK, Pardemann R, Meyer B (2016) Estimating the global waste heat potential. Renew Sustain Energy Rev 57:1568–1579. https://doi.org/10.1016/J.RSER.2015.12.192
Bianchi G, Panayiotou GP, Aresti L, Kalogirou SA (2019) Estimating the waste heat recovery in the European Union Industry. Energy Ecol Environ. https://doi.org/10.1007/s40974-019-00130-9
Making the right choice–organic rankine cycle compared to steam cycle. https://www.host.nl/en/orc-vs-steamcycle/
Marchionni M, Bianchi G, Tsamos KM, Tassou SA (2017) Techno-economic comparison of different cycle architectures for high temperature waste heat to power conversion systems using CO2 in supercritical phase. Energy Procedia 123:305–312. https://doi.org/10.1016/j.egypro.2017.07.253
Ahn Y, Lee JI, Lee JI (2016) A study of s-CO2 power cycle for waste heat recovery using isothermal compressor. Vol. 9 Oil Gas Appl. Supercrit. CO2 Power Cycles; Wind Energy, vol 9, American Society of Mechanical Engineers (ASME), p V009T36A012. https://doi.org/10.1115/gt2016-57151
Turchi CS, Ma Z, Dyreby J (2012) Supercritical carbon dioxide power cycle configurations for use in concentrating solar power systems. Proc ASME Turbo Expo 5:967–973. https://doi.org/10.1115/GT2012-68932
Conboy TM, Wright SA, Ames DE, Lewis TG (2012) CO2-based mixtures as working fluids for geothermal turbines. Sandia National Laboratories, Albuquerque. https://doi.org/10.2172/1049477
Bianchi G, Saravi SS, Loeb R, Tsamos KM, Marchionni M, Leroux A (2019) Design of a high-temperature heat to power conversion facility for testing supercritical CO2 equipment and packaged power units. Energy Procedia 161:421–428. https://doi.org/10.1016/J.EGYPRO.2019.02.109
Moisseytsev A, Sienicki JJ (2009) Investigation of alternative layouts for the supercritical carbon dioxide Brayton cycle for a sodium-cooled fast reactor. Nucl Eng Des 239:1362–1371. https://doi.org/10.1016/j.nucengdes.2009.03.017
Johnson G, McDowell M (2009) Issues associated with coupling supercritical CO2 power cycles to nuclear, solar and fossil fuel heat sources. In: Proceedings of supercrit CO2 power cycle 2009
Le Moullec Y (2013) Conceptual study of a high efficiency coal-fired power plant with CO2 capture using a supercritical CO2 Brayton cycle. Energy. https://doi.org/10.1016/j.energy.2012.10.022
Allam R, Martin S, Forrest B, Fetvedt J, Lu X, Freed D et al (2017) Demonstration of the Allam Cycle: an update on the development status of a high efficiency supercritical carbon dioxide power process employing full carbon capture. Energy Procedia 114:5948–5966. https://doi.org/10.1016/j.egypro.2017.03.1731
Sabau A, Yin H, Qualls A, McFarlane J (2011) Investigations of supercritical CO2 Rankine cycles for geothermal power plants. Oak Ridge National Lab, Oak Ridge
Turchi CS, Ma Z, Neises TW, Wagner MJ (2013) Thermodynamic study of advanced supercritical carbon dioxide power cycles for concentrating solar power systems. J Sol Energy Eng 135:41007. https://doi.org/10.1115/1.4024030
Iverson BD, Conboy TM, Pasch JJ, Kruizenga AM (2013) Supercritical CO2 Brayton cycles for solar-thermal energy. Appl Energy 111:957–970. https://doi.org/10.1016/j.apenergy.2013.06.020
Hung T (2001) Waste heat recovery of organic Rankine cycle using dry fluids. Energy Convers Manag. https://doi.org/10.1016/S0196-8904(00)00081-9
Dai X, Shi L, Qian W (2019) Review of the working fluid thermal stability for organic rankine cycles. J Therm Sci 28:597–607. https://doi.org/10.1007/s11630-019-1119-3
Zywica G, Kaczmarczyk TZ, Ihnatowicz E (2016) A review of expanders for power generation in small-scale organic Rankine cycle systems: performance and operational aspects. Proc Inst Mech Eng Part A J Power Energy 230:669–684. https://doi.org/10.1177/0957650916661465
Weiß AP (2015) Volumetric expander versus turbine–which is the better choice for small Orc plants. In: 3rd international seminar on ORC power systems, Oktober (pp. 12–14)
Tartière T, Astolfi M (2017) A world overview of the organic rankine cycle market. Energy Procedia 129:2–9. https://doi.org/10.1016/j.egypro.2017.09.159
Weiß, A. P. (2015). Volumetric expander versus turbine–which is the better choice for small ORC plants. In 3rd International Seminar on ORC Power Systems, Oktober (pp. 12-14)
Tanuma T (2016) Advances in steam turbines for modern power plants. Elsevier Inc, Amsterdam
Li C, Wang H (2016) Power cycles for waste heat recovery from medium to high temperature flue gas sources —from a view of thermodynamic optimization. Appl Energy 180:707–721. https://doi.org/10.1016/j.apenergy.2016.08.007
Smith IK (1993) Development of the trilateral flash cycle system: part 1: fundamental considerations. Proc Inst Mech Eng Part A J Power Energy 207:179–194. https://doi.org/10.1243/PIME_PROC_1993_207_032_02
Bianchi G, McGinty R, Oliver D, Brightman D, Zaher O, Tassou SA et al (2017) Development and analysis of a packaged trilateral flash cycle system for low grade heat to power conversion applications. Therm Sci Eng Prog 4:113–121. https://doi.org/10.1016/J.TSEP.2017.09.009
Marchionni M, Zaher O, Miller J (2019) Numerical investigations of a trilateral flash cycle under system off-design operating conditions. Energy Procedia 161:464–471. https://doi.org/10.1016/J.EGYPRO.2019.02.070
Kung SC, Shingledecker JP, Thimsen D, Wright IG, Tossey BM, Sabau AS (2016) Oxidation/corrosion in materials for supercritical CO2 power cycles
Marchionni M, Bianchi G, Tassou SA (2018) Techno-economic assessment of Joule–Brayton cycle architectures for heat to power conversion from high-grade heat sources using CO2 in the supercritical state. Energy 148:1140–1152. https://doi.org/10.1016/J.ENERGY.2018.02.005
De Miol M, Bianchi G, Henry G, Holaind N, Tassou SA, Leroux A (2018) Design of a single-shaft compressor, generator, turbine for small-scale supercritical CO2 systems for waste heat to power conversion applications. https://doi.org/10.17185/duepublico/46086
Mehos M, Turchi CS, Jorgenson J, Denholm P, Ho C, Armijo K (2016) On the path to sunshot-advancing concentrating solar power technology. Perform Dispatchability. https://doi.org/10.2172/1344199
Held TJ 2014 Initial test results of a megawatt-class supercritical CO2 heat engine. In: 4th international symposium CO2 power cycles
Bachu S, Freund P, Gupta M, Simbeck D, Thambimuthu K (2005) Annex I: properties of CO2 and carbon-based fuels. IPCC special report on carbon dioxide capture and storage. Cambridge University Press, New York
Angelino G (1968) Carbon dioxide condensation cycles for power production. ASME J Eng Power 90:287–296. https://doi.org/10.1115/1.3609190
Kacludis A, Lyons S, Nadav D, Zdankiewicz E (2012) Waste heat to power (WH2P) applications using a supercritical CO2-based power cycle. Power-Gen Int 2:1–10
Persichilli M, Kacludis A, Zdankiewicz E, Held T (2012) Supercritical CO2 power cycle developments and commercialization: why sCO2 can displace steam steam. Power-Gen India Cent Asia 2012:19–21
Nami H, Mahmoudi SMS, Nemati A (2017) Exergy, economic and environmental impact assessment and optimization of a novel cogeneration system including a gas turbine, a supercritical CO2 and an organic Rankine cycle (GT-HRSG/SCO2). Appl Therm Eng 110:1315–1330. https://doi.org/10.1016/j.applthermaleng.2016.08.197
Wright SA, Davidson CS, Scammell WO (2016) Thermo-economic analysis of four sCO2 waste heat recovery power systems
Huck P, Freund S, Lehar M, Peter M (2016) Performance comparison of supercritical CO2 versus steam bottoming cycles for gas turbine combined cycle applications
Saari H, Petrusenko R, Zanganeh K, Parks C, Maybee B (2014) Corrosion testing of high temperature materials in supercritical carbon dioxide. In: 4th international symposium supercritical CO2 power cycles, pp 1–13
Yin H, Sabau AS, Conklin JC, McFarlane J, Lou Qualls A (2013) Mixtures of SF6-CO2 as working fluids for geothermal power plants. Appl Energy 106:243–253. https://doi.org/10.1016/j.apenergy.2013.01.060
Jeong WS, Jeong YH (2013) Performance of supercritical Brayton cycle using CO2-based binary mixture at varying critical points for SFR applications. Nucl Eng Des 262:12–20. https://doi.org/10.1016/j.nucengdes.2013.04.006
Wright SA, Radel RF, Vernon ME, Rochau GE, Pickard PS (2010) Operation and analysis of a supercritical CO2 Brayton Cycle. https://doi.org/10.2172/984129
Brun K, Friedman P, Dennis R (2017) Fundamentals and applications of supercritical carbon dioxide (sCO2) based power cycles. Woodhead Publishing an imprint of Elsevier, Amsterdam
Beckman K, Patel V (2000) Review of API versus AGMA gear standards/rating, data sheet completion, and gear selection guidelines. In: Proceeding of the twenty-ninth
Wilkes J, Allison T, Schmitt J, Bennett J. Application of an integrally geared compander to an SCO2 recompression Brayton cycle. In: Proceeding 5th international symposium 2016
Kalra C, Hofer D, Sevincer E, Moore J, Brun K (2014) Development of high efficiency hot gas turbo-expander for optimized CSP supercritical CO2 power block operation. In: The 4th international symposium-supercritical CO2 power cycles
Ertas B (2009) Compliant hybrid journal bearings using integral wire mesh dampers. In: Turbo Expo Power Land, Sea, Air, p 12. https://doi.org/10.1115/gt2008-50984
Delgado A (2015) Experimental identification of dynamic force coefficients for a 110 mm compliantly damped hybrid gas bearing. J Eng Gas. https://doi.org/10.1115/1.4029203
Thatte A, Loghin A, Shin Y, Ananthasayanam B (2016) Performance and life characteristics of hybrid gas bearing in a 10 MW supercritical CO2 turbine. Vol. 9 Oil Gas Appl. Supercrit. CO2 Power Cycles; Wind Energy, ASME, p V009T36A018. https://doi.org/10.1115/gt2016-57695
Sienicki J, Moisseytsev A, Fuller R, Wright S (2011) Scale dependencies of supercritical carbon dioxide Brayton cycle technologies and the optimal size for a next-step supercritical CO2 cycle. 2011 Supercrit CO2
Thatte A, Dheeradhada V (2016) Coupled physics performance predictions and risk assessment for dry gas seal operating in MW-scale supercritical CO2 turbine. Vol. 9 Oil Gas Appl. Supercrit. CO2 Power Cycles; Wind Energy, ASME, p V009T36A017. https://doi.org/10.1115/gt2016-57670
Bidkar RA, Sevincer E, Wang J, Thatte AM, Mann A, Peter M et al (2016) Low-leakage shaft-end seals for utility-scale supercritical CO2 turboexpanders. J Eng Gas Turbines Power 139:22503. https://doi.org/10.1115/1.4034258
Pasch J, Conboy T, Fleming D, Rochau G (2012) Supercritical CO2 Recompression Brayton Cycle: Completed Assembly. Sandia Report, SAND, 9546, 2012
Muhammad HA, Lee B, Lee G, Cho J, Baik Y-J (2018) Investigation of leakage reinjection system for supercritical CO2 power cycle using heat pump. Renew Energy. https://doi.org/10.1016/J.RENENE.2018.10.059
Lettieri C, Baltadjiev N, Casey M, Spakovszky Z (2014) Low-flow-coefficient centrifugal compressor design for supercritical CO2. J Turbomach 136:81008. https://doi.org/10.1115/1.4026322
Baltadjiev ND, Lettieri C, Spakovszky ZS (2015) An investigation of real gas effects in supercritical CO2 centrifugal compressors. J Turbomach 137:91003. https://doi.org/10.1115/1.4029616
Poerner M, Musgrove G, Beck G (2016) Liquid CO2 formation, impact, and mitigation at the inlet to a supercritical CO2 compressor. Vol. 9 Oil Gas Appl. Supercrit. CO2 Power Cycles; Wind Energy, ASME, p V009T36A005. https://doi.org/10.1115/gt2016-56513
Clementoni EM, Cox TL, King MA, Rahner KD (2017) Transient power operation of a supercritical carbon dioxide brayton cycle. Vol. 9 Oil Gas Appl. Supercrit. CO2 Power Cycles; Wind Energy, ASME, p V009T38A001. https://doi.org/10.1115/gt2017-63056
Clementoni EM, Cox TL, Sprague CP (2014) Startup and operation of a supercritical carbon dioxide brayton cycle. J Eng Gas Turbines Power 136:V008T34A006. https://doi.org/10.1115/gt2013-94275
Moore J, Evans N, Kalra C, Brun K, Director P, Bueno P et al (2015) Development of A1 MWE supercritical CO2 Brayton cycle test loop. ASME Turbo Expo 2015 turbine technical conference exposition, p 11. https://doi.org/10.1115/GT2015-43771
Cho J, Shin H, Cho J, Baik YJ, Choi B, Roh C, et al (2018) Design, flow simulation, and performance test for a partialadmission axial turbine under supercritical CO2 condition. In: Proceedings of the ASME turbo expo, vol 9. American Society of Mechanical Engineers (ASME). https://doi.org/10.1115/gt2018-76508
Cha JE, Bae SW, Lee J, Cho SK, Lee JI, Park JH (2016) Operation results of a closed supercritical CO2 simple Brayton cycle. In: 5th international symposium CO2 power cycles
Cho J, Shin H, Ra HS, Lee G, Roh C, Lee B, et al. (2016) Development of the supercritical carbon dioxide power cycle experimental loop in kier.In: Proceedings of the ASME turbo expo, vol 9. American Society of Mechanical Engineers (ASME). https://doi.org/10.1115/gt2016-57460
De Miol M, Bianchi G, Henry G, Holaind N, Tassou SA, Leroux A (2018) Design of a single-shaft compressor, generator, turbine for small-scale supercritical CO2 systems for waste heat to power conversion applications. In: 2nd European sCO2 conference 2018: 30–31 August 2018, Essen, Germany, pp 42–49. https://doi.org/10.17185/duepublico/46086
Hacks A, Schuster S, Dohmen HJ, Benra FK, Brillert D (2018) Turbomachine design for supercritical carbon dioxide within the sCO2-HeRo.eu project. J Eng Gas Turbines Power 10(1115/1):4040861
Utamura M, Hasuike H, Ogawa K, Yamamoto T, Fukushima T, Watanabe T et al (2012) Demonstration of supercritical CO2 closed regenerative Brayton cycle in a bench scale experiment. Proc ASME Turbo Expo 3:155–164. https://doi.org/10.1115/GT2012-68697
Marchionni M, Chai L, Bianchi G, Tassou SA (2019) Numerical modelling and transient analysis of a printed circuit heat exchanger used as recuperator for supercritical CO2 heat to power conversion systems. Appl Therm Eng 161:114190. https://doi.org/10.1016/j.applthermaleng.2019.114190
Sienicki JJ, Moisseytsev A, Lv Q (2017) Dry air cooling and the sCO2 brayton cycle. Vol. 9 Oil Gas Appl. Supercrit. CO2 Power Cycles; Wind Energy, vol. 9, American Society of Mechanical Engineers (ASME), p V009T38A015. https://doi.org/10.1115/gt2017-64042
Brun K, Friedman P, Dennis R (2017) Fundamentals and applications of supercritical carbon dioxide (sCO2) based power cycles. Woodhead Publishing, Sawston
Musgrove GO, Pierres R Le, Nash J (2014) Heat exchangers for supercritical CO2 power cycle applications. In: 4th international symposium-supercritical CO2 power cycles, pp 1–61
Musgrove GO, Sullivan S, Shiferaw D, Pittaway C, Carrero J, Le Pierres R et al (2014) Heat exchangers for supercritical CO2 power cycle applications tutorial
Carlson M, Kruizenga AM, Schalansky C, Fleming DF (2014) Sandia progress on advanced heat exchangers for sCO2 Brayton cycles.In: 4th international symposium-supercritical CO2 power cycles
Marchionni M, Bianchi G, Karvountzis-Kontakiotis A, Pesyridis A, Tassou SA (2018) An appraisal of proportional integral control strategies for small scale waste heat to power conversion units based on organic Rankine cycles. Energy 163:1062–1076. https://doi.org/10.1016/J.ENERGY.2018.08.156
Fourspring PM, Nehrbauer JP, Sullivan S, Nash J (2014) Testing of compact recuperators for a supercritical CO2 Brayton power cycle. In: 4th international symposium-supercritical CO2 power cycles
Parks CJ (2013) Corrosion of candidate high temperature alloys in supercritical carbon dioxide. Doctoral dissertation, Carleton University
Moore R, Conboy T (2012) Metal corrosion in a supercritical carbon dioxide–liquid sodium power cycle. https://doi.org/10.2172/1039408
Rouillard F, Charton F, Moine G (2011) Corrosion behavior of different metallic materials in supercritical carbon dioxide at 550 C and 250 bars. Corros J Sci Eng. https://doi.org/10.5006/1.3628683
Dunlevy MW (2009) An exploration of the effect of temperature on different alloys in a supercritical carbon dioxide environment. Doctoral dissertation, Massachusetts Institute of Technology
Gibbs, JP (2010) Corrosion of various engineering alloys in supercritical carbon dioxide. Doctoral dissertation, Massachusetts Institute of Technology
Sridharan K, Anderson M (2013) Corrosion in supercritical carbon dioxide: materials, environmental purity, surface treatments, and flow issues (No. 10-872). Battelle Energy Alliance, LLC. https://doi.org/10.2172/1111547
Poláčková J, Petrů J, Janák M, Berka J, Krausová A (2017) Materials for use in calcium looping technology for CCS–corrosion processes in high-temperature CO2. Koroze a ochrana materialu 61(4):143–148. https://doi.org/10.1515/kom-2017-0017
Lim JY, McKrell TJ, Eastwick G, Ballinger RG (2008) Corrosion of materials in supercritical carbon dioxide environments. In: Corros. NACE International corrosion conference Expo, 63. NACE International, p 18
Clementoni EM, Cox TL (2014) Steady-state power operation of a supercritical carbon dioxide brayton CYCLE. In: 4th International Symposium on Supercritical CO2 power cycles. https://doi.org/10.1115/gt2014-25336
Cho J, Shin H, Cho J, Choi B, Roh C, Lee B et al (2019) Development and power generating operation of the supercritical carbon dioxide power cycle experimental test loop in Kier. https://doi.org/10.17185/duepublico/48905
Hacks AJ, Vojacek A, Dohmen HJ, Brillert D, Vojacek A et al (2018) Experimental investigation of the sCO2-HeRo compressor. https://doi.org/10.17185/duepublico/46088
Vojacek A, Hacks AJ, Melichar T, Frybort O, Hájek P (2018) Challenges in supercritical CO2 power cycle technology and first operational experience at CVR. https://doi.org/10.17185/duepublico/46075
Acknowledgements
The research presented in this paper has received funding from the European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 680599. The manuscript reports all the relevant data to support the understanding of the results. More detailed information and data, if required, can be obtained by contacting the corresponding author of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marchionni, M., Bianchi, G. & Tassou, S.A. Review of supercritical carbon dioxide (sCO2) technologies for high-grade waste heat to power conversion. SN Appl. Sci. 2, 611 (2020). https://doi.org/10.1007/s42452-020-2116-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2116-6