Abstract
Textile exchange is a popular term in the textile industry and has incorporated Sustainable Development Goals (SDGs) into its strategy for transformation. The advance of Industrial Revolution 4.0 digitised the textile industry and incorporated artificial intelligence (AI) into operation and supply chain to enhance production and improve product quality in different downstream processing. Malaysia’s textile industry has largely continued to contribute to the domestic economy. In the textile industry, refinement treatment is crucial to avoid water pollution derived from dye, organic pollutants, and heavy metals. Wastewater derived from textile industries must perform a pre-treat before discarding into the natural environment, as the excess dye in textile wastewater negatively impacts the environment. Untreated or inadequately treated wastewater and discharge to nearby water sources contribute to disease increase. Therefore, this study aims to elucidate microbiota biodiversity that can significantly remove or break down the dye in wastewater from the textile industry. This study selected two cationic dyes, crystal violet (CV), and methylene blue (MB). Through the findings, microbial consortium derived from the fermented bio-waste show no significant difference in the decolourisation of wastewater polluted with CV (< 13.53%). In contrast, the microbial population generated from fermented bio-waste showed remarkable outcomes on MB decolourisation up to 88.52% in 3 days of treatment. In the absence of laccase in fermented bio-waste, microbial consortium produced from the fermented bio-waste is ineffective in decolourising the wastewater containing CV compared to MB.
Similar content being viewed by others
Introduction
The textile industry is one of Malaysia’s rapidly developing industries and significantly impacts the country’s economic development. Malaysia’s textiles and clothing industry’s GDP contribution is approximately 1.2% [1]. However, environmental deterioration might occur intentionally or accidentally by discharging untreated wastewater with high pollution loads to nearby water sources during the processing. Recently, environmentalists focused on utilising high and advanced technology to create a filtration system to remove the dyes. But due to the high cost involved, the textile industry’s willingness to implement the system is still considered limited. Recycling material or waste has become a new research brand to promote sustainability. Studies on using lower-cost materials with more effective dye removal were carried out, such as using living organisms for bio-waste [2,3,4] to remove the dye in the wastewater.
According to the Department of Environment (DOE), 54% of the assessed rivers in Malaysia were classified as slightly polluted and polluted in 2017. Goi (2020) analysed the industrial park in Selangor, Johor, Penang and Perak, Malaysia, and the high pollution level of river waters contributed [5]. As a result, water pollution is alarming and becoming a concerning issue because it will directly affect the sustainability of the community [6]. Thus, wastewater generated should be treated more effectively to improve water quality before it is discharged into the environment. Meanwhile, implementing efficient wastewater treatment can also accomplish Sustainable Development Goal (SDG) 6, which emphasises water quality and sanitation.
Besides fibres and chemicals, dyes in wastewater are considered pollutants that can harm the environment and the health of living organisms. The diverse composition of pigments, including azo, triphenylmethane, and anthraquinone, is recalcitrant and considered pollutants in water due to their toxic and chromophoric properties [7]. The dye or its degraded intermediates with specific chemical structures like the azo group are potentially mutagenic and carcinogenic to aquatic life. Some dyes with good bioaccumulation and biomagnification properties might even possess hazardous effects across the entire ecosystem’s food chain, including human health [8]. Furthermore, dye’s intense colour will alter water’s physical properties even at low concentrations by inhibiting sunlight penetration and disrupting light absorption by photosynthetic plants, thereby leading to severely impaired photosynthetic processes in an aquatic ecosystem [9]. In addition, the presence of dye in water will indirectly reduce the level of dissolved oxygen as the oxygen supplied by the photosynthetic plants under the water is influenced by the light intensity that can penetrate the water.
Crystal violet dye (CV) is used as one of the colouring materials and is a dark purple synthetic triaryl methane colouring [10]. It is used for painting in the textile industry. However, this dye is toxic in various ways. On the other hand, methylene blue (MB) is a heterocyclic aromatic chemical compound that yields a blue solution when dissolved in water. The formulas for CV and MB are C25H30ClN3 and C16H18ClN3S, respectively. Dye removal methods can be separated into three main categories, biological, chemical, and physical [11,12,13]. Natural treatment using bacterial decolourisation has been reported as an economical alternative to chemical and physical treatment. Biodegradation methods such as fungal decolourisation, microbial degradation, adsorption by living or dead microbiota biomass, and bioremediation systems are commonly applied to treat industrial effluents because many microorganisms such as bacteria, yeasts, algae, and fungi can accumulate and degrade different pollutants [11,12,13,14,15].
Thus, this study will investigate the efficiency of microbial consortium derived from fermented bio-waste, including fruit and vegetable waste, to remove CV and MB in synthetic wastewater. This data is essential to serve as preliminary findings to design a cost-effective and environmentally friendly method to decolourise CV and MB. With this, researchers wish to increase the public’s awareness of recycling waste with proven beneficial impacts on environmental bioremediation.
Material and Method
Fermentation of Bio-Waste
The fermentation medium for bio-waste was prepared according to the composition described by Arun and Sivashanmugam (2017) [16]. Bio-waste in the labelled container was mixed sufficiently with other ingredients according to the composition ratio stated in 3:1:10 (Waste: brown sugar: distilled water). Wastes from local households contain peels and seeds of mixed fruits like oranges, lemons, and apples and labelling as household eco-enzyme (H-EE). In contrast, fruit waste collected from a vendor at block G, UCSI University, was mainly composed of pomaces and dregs from carrots, papaya, pineapple and orange, labelling as vendor eco-enzyme (V-EE). The collected fruit waste was washed thoroughly with distilled water to remove dirt and debris. Since the fermentation of bio-waste for eco-enzyme production depends on the native microbes present in it, thus, the bio-waste was not subjected to drying or sterilisation to preserve the native microbial population. H-EE and V-EE were produced via anaerobic fermentation for 4 and 12 months, respectively.
Synthetic Wastewater Treatment by Eco-Enzyme
Characterisation of Eco-Enzyme Derived from Bio-Waste
The extracted eco-enzyme from H-EE and V-EE were characterised by the quantification of pH, total dissolved solids (TDS), Ca2+, Na+, K+, and NO3− with Horiba LAQUAtwin water quality probes. Concentrations NH4+ and PO43− were also determined via colourimetric assay with API® Ammonium and Phosphate Test Kit. Different concentrations of each eco-enzyme (H-EE and V-EE) were prepared by diluting harvested eco-enzyme with distilled water. A 100 mL eco-enzyme with four different concentrations (v/v) was prepared to a final concentration of 5%, 10%, and 20% (v/v).
Preparation of Synthetic Wastewater
According to Quilliam et al. (2020) [17], coronavirus, known as SARS-Cov-2, was detected in untreated sewerage and contaminated natural water bodies. This might increase the infection risk of COVID-19 disease during the collection or handling of wastewater. Hence, untreated wastewater sample was not collected from the sewerage treatment plants to prevent undesired infections. Instead, the treatment of actual wastewater was substituted by synthetic wastewater prepared in this study. Synthetic wastewater was prepared using OECD synthetic sewage method. The medium was prepared by adding peptone; 160 mg of meat extract; 110 mg urea; 30 mg anhydrous dipotassium hydrogen phosphate (K2HPO4); 28 mg sodium chloride (NaCl); 7 mg calcium chloride dihydrate (CaCl2.2H2O); 4 mg and magnesium sulphate heptahydrate (MgSO4.7H2O); and 2 mg in 1 L of tap water. This synthetic wastewater was concentrated ten times with dissolved organic carbon (DOC) concentration in 100 mg/L, which is considered closer to real sewage [18]. The prepared synthetic wastewater was kept in a closed area for over 1 week before treatment by eco-enzyme to stabilise chemicals within the synthetic wastewater. It was also noted that synthetic wastewater was characterised before treatment by evaluating its water quality parameters as described in “water quality testing of treated wastewater”.
Treatment of Wastewater Sample by Eco-Enzyme
Synthetic wastewater and distilled water were divided into aliquots of 40 mL samples in the falcon tubes. Then, 10 mL of the eco-enzyme solution of H-EE were added accordingly into each aliquot to achieve a final concentration of eco-enzyme of 5%, 10%, and 20% (v/v), respectively. The procedures above were repeated by using eco-enzyme harvested from V-EE labelled containers. In this experiment, negative control of overall treatment by eco-enzyme was prepared by substituting 10 mL of eco-enzyme with distilled water. On the other hand, negative control of each eco-enzyme concentration was also designed compared to the effect of eco-enzyme on synthetic wastewater. Thus, the wastewater was replaced with distilled water.
Water Quality Testing of Treated Wastewater
Wastewater treatment by eco-enzyme was conducted for a duration of 5 days. The parameters’ changes of each treated sample were evaluated to identify the effect of eco-enzyme on the selected parameters. The parameters chosen in this study were the concentration of sodium ions (Na+), potassium ions (K+), calcium ions (Ca2+), nitrate ions (NO3−), phosphate ions (PO43−), ammonium ions (NH4+), total dissolved solids (TDS) and pH value by using Horiba LAQUAtwin water quality probe. These parameters were measured daily throughout the 5 days of treatment. Whilst NH4+ and PO43− were measured from day 1 to day 5 via the colourimetric method by UV/Vis spectrophotometer. The colourimetric assay, API® Ammonium and Phosphate Test Kit enables colour development before spectrophotometric measurement.
A standard curve of OD650 against NH4+ concentration and OD725 against PO43− was plotted accordingly to obtain the equation [19, 20].
Dye Decolourization Assay
A dye decolourisation assay was carried out to evaluate the capabilities of fermented bio-waste on dye decolourisation. The decolourisation method was modified based on Maya et al. (2019) [21].
Preparation of Stock Solution
Phosphate buffer (100 mM, pH 6.4) was prepared by mixing 132.5 mL of 0.2 M Na2HPO4 and 367.5 mL of 0.2 M NaH2PO4, followed by the addition of distilled water to make a final volume of 1 L. Next, a stock solution of crystal violet (CV) with an absorbance range of 0.5–0.9 was prepared by mixing phosphate buffer and CV powder. The liquid solution was then filtered to remove any suspension and solid residues. Subsequently, the answer was divided into aliquots of 8 mL in each capped and labelled glass bottle, followed by autoclave sterilisation to eliminate microorganisms that may interfere with the accuracy of the decolourisation assay. The above procedures were repeated to prepare a methylene blue (MB) stock solution. It was noted that the units for the concentration of the MB solution were millilitre/litre (mL/L) as the phosphate buffer was mixed with a solution of methylene blue. Furthermore, standard curves for each dye were also plotted.
Determination of Decolourisation Efficiency
The assay was carried out where 2 mL of fermented bio-waste were added into each aliquot of dye solution to achieve final concentrations of 5% (HA or VA), 10% (HB or VB), and 20% (HC or VC) (v/v), respectively. The mixture was incubated in a dark area at room temperature for 3 days to prevent photodegradation or photo-decolourization of the dye. The absorbance of CV at 590 nm was measured on day 1 and day 3 using a UV/Vis spectrophotometer [22]. Subsequently, the absorbance value obtained was converted into its respective concentration using the standard curve plotted and recorded. This procedure was repeated with a decolourisation assay on MB. As the methods of dye decolourisation assay were carried out, it was noted that the wavelength used for absorbance measurement of MB was 668nm [23]. A relative dye decolourisation efficiency was determined through the equation below to evaluate the efficiency of fermented bio-waste on dye decolourisation [24].
where Ci and Cf are the dye’s initial and final concentrations, respectively.
Statistical Analysis
The present study’s findings were analysed using a t-test performed on all the treatments and done using the Statistical Package for Social Sciences (SPSS) version 9.0 to determine the significance of experimental studies of dye decolourisation.
Results and Discussion
Physiochemical Characterisation of Eco-Enzyme in Fermented Bio-Waste
Environmental pollution is irreversible. Although many researchers try to use different approaches to clean up and break down the pollutant, the researcher needs to investigate whether the newly developed method brings other harmful side effects to the environment before this method applies. In this study, fermented bio-waste was tested in wastewater to identify its impact on the wastewater.
The characterisation of the H-EE and V-EE were quantitated according to the environmental parameters selected. The initial concentration of sodium ions (Na+), potassium ions (K+), calcium ions (Ca2+), nitrate ions (NO3−), phosphate ions (PO43−), and ammonium ions (NH4+), total dissolved solids (TDS) in parts per million (mg/L), as well as pH of both eco-enzymes, were tabulated together in Table 1.
According to Table 1, the pH of H-EE and V-EE, which were less than 7, indicated that the EE produced from anaerobic fermentation is acidic. The acidic properties of both EE are attributed to the presence of various volatile and organic acids [25]. For instance, acetic acid is the product of complete anaerobic fermentation, where microorganisms break down complex organic compounds. Other types of organic acid like citric acid, oxalic acid, lactic acid, and malic acid were also present in EE solution at lower concentrations than acetic acid [26, 27].
During fermentation, microorganisms will break down bio-waste as energy sources. Fermentation can improve mineral contents as microorganisms in the bio-waste and enable the enzyme production for amino-acid in plant-based food waste [28]. Mineral elements like Na+, K+, and Ca2+ within the extracted EE were also evaluated. Based on Table 1, the Na+, K+, and Ca2+ between H-EE and V-EE were significantly different (P < 0.05), where H-EE had a lower concentration of inorganic ions than V-EE. These dissolved mineral elements originated from the sample of organic waste valorised for EE production. According to the studies by Czech et al. (2020) and Sharma et al. (2012), the peels of citrus fruits and apples used in H-EE production were found to have less mineral composition of sodium, potassium, and calcium as compared to carrot pulp in V-EE production [29, 30].
Moreover, the longer duration of fermentation, the more the mineral elements will be released from the complex molecules in bio-waste via decomposition. Feyera and teams (2020) [31] reported that increasing fermentation duration could enhance zinc and vitamin percentages. Hence, V-EE with 12 months of fermentation age is said to have a higher concentration of Na+, K+, and Ca2+ than 4 months old of H-EE.
High TDS values were detected for both extracted EE due to the substrate’s abundant dissolved organic matter, like brown sugar and bio-waste in the fermentation medium [32]. According to Ademollo et al. (2012) [33], organic matter in solution is partitioned between phases of suspended and dissolved solids. The solubilised organic matter, therefore, causes a high TDS value of both EE. Moreover, electrolytes like dissolved minerals and organic acids in EE also presumably induced high TDS, as was proven by the high concentration of Na+ and K+ in extracted EE. Table 1 shows that the concentration of NO3− in H-EE and V-EE was 97.5 mg/L and 87.52 mg/L, respectively. Whilst NH4+ in H-EE and V-EE was detected at low concentration, denoted by 1.42 mg/L and 1.47 mg/L, respectively. Both NO3− and NH4+ are inorganic nitrogenous compounds involved in the nitrogen cycle, and they originated from nitrogen sources like protein, amino acids and nucleic acids present in the fruit waste. EE contains various active enzymes, such as amylase, lipase, and protease, produced by the inoculated microbes during fermentation [16]. Proteolytic enzymes from decomposers play a role in degrading the organic nitrogenous compounds into inorganic ammonia via ammonification.
Interestingly, nitrification also converts ammonia into nitrates by nitrifying bacteria, thereby contributing to the nitrate content in the EE solution [34]. H-EE with a higher concentration of NO3− but a lower concentration of NH4+ implied that more nitrifying bacteria might present in H-EE than V-EE. Therefore, it might eventually result in high nitrification activity.
The PO43− concentration of H-EE and V-EE was 0.96 mg/L and 31.24 mg/L, respectively, and they were statistically significantly different as the p-value is less than 0.05. V-EE has higher PO43− than H-EE as its fermented duration is longer than H-EE. During fermentation, phosphorus-rich biological compounds from bio-waste like nucleic acids and plasma membrane will be degraded by microbial enzymes and contribute to the PO43− content in EE. As the fruit waste is fermented longer, more inorganic PO43− will be released upon the degradation of biomolecules by microbes. Another reason for such an outcome might be the high propionic acid or acetic acid content in H-EE with lower pH, as the propionate and acetate are utilisable by the biomass of polyphosphate-accumulating organisms (PAO) as carbon sources. In return, the growth of PAO like Acinetobacter spp. and Lampropedia spp. is highly encouraged by the presence of acetate in H-EE, eventually leading to high phosphorus uptake and storage as polyphosphate by these PAO [35, 36].
Water Quality of Synthetic Wastewater with Bio-Waste
This study conducted a 5 days treatment to evaluate eco-enzyme efficiency and influences on synthetic wastewater. Nazim and Meera (2013) reported that the optimum duration of wastewater treatment by eco-enzymes is 5 days as a remarkable improvement in the water quality of greywater was demonstrated after 5 days of treatment, where chemical oxygen demand (COD) and biochemical oxygen demand (BOD) were reduced by 50% and 69%, respectively [37]. The significant removal efficiency of TSS in dairy wastewater also indicated that the positive effects of eco-enzyme in enhancing water quality were getting significant after 5 days of treatment [38]. On the other hand, different concentrations of each eco-enzyme, denoted by 5% (v/v), 10% (v/v) and 20% (v/v), were adopted for wastewater treatment in this study. Studies conducted by Tang and Tong (2011) [39] recommended concentration of 10 times dilution is effective in water purification. Thus, higher and lower concentrations of eco-enzyme from recommended concentrations in wastewater treatment were also evaluated to determine the optimum concentrations.
Several parameters were measured daily throughout the 5 days treatment to evaluate the influence of the eco-enzyme on water quality. The parameters were pH, total dissolved solids (TDS), concentrations of ions like sodium ions (Na+), potassium ions (K+), calcium ions (Ca2+), nitrate ions (NO3−), phosphate ions (PO43−), and ammonium ions (NH4+). The concentration of inorganic ions such as Na+, K+, and Ca2+ was evaluated to determine the influence of eco-enzyme on electroconductivity and wastewater salinity as there are minimal findings about the variation of inorganic ions upon the wastewater treatment by eco-enzyme. TDS, which measures the contents of all dissolved organic and inorganic substances in water, was also determined as it reflects more comprehensive information about water salinity [40]. Whereas parameters like NO3, NH4+, and PO43− were determined to evaluate the capabilities of eco-enzyme in reducing the nutrients load in wastewater, thereby preventing the incidence of water pollution, which resulted from eutrophication due to high nutrient loading in discharged wastewater [41]. Table 2 shows the concentration of different ions in and pH of the synthetic wastewater before treatment. Table 3 illustrates the daily variation of concentration of other ions, TDS, and pH values in synthetic wastewater samples treated with eco-enzymes and a control sample, which was untreated wastewater.
According to Environmental Quality (Sewage) Regulations 2009, the pH of synthetic wastewater was close to the permissible range of effluent standards of 5.5–9.0. As Meiramkulova et al. (2022) reported the pH of synthetic wastewater prepared using distilled water is acidic (pH 5.5). In this study, the pH of synthetic wastewater is 5.55 ± 0.49. This indicates nitrification occurs before treatment as synthetic wastewater was prepared in a none sterile condition in the laboratory at room temperature (28–32 °C) [42].
The World Health Organization (WHO) reported that the discharged standard for sodium and calcium should be less than 200 mg/L, and the concentration of sodium ions in synthetic wastewater has exceeded the acceptable level. Whereas the potassium level in natural freshwater systems should be less than 10 mg/L, the potassium level of 33.11 mg/L in synthetic wastewater is also beyond the limits. By the standard of permissible effluent established by the Department of Environment (DOE), the prepared wastewater had surpassed the allowable limit of nitrate and ammoniacal nitrogen level of less than 20 mg/L and 5 mg/L, respectively. Moreover, 5.66 mg/L of phosphate in the synthetic wastewater exceeded the permissible range of 1–2 mg/L of total phosphorus.
Furthermore, the TDS level of synthetic wastewater was also beyond the limit of 500 mg/L as established by the Bureau Indian of Standards. Thus, it was known that the water quality of prepared synthetic wastewater deteriorated up to the polluted range. Hence, the wastewater requires treatment as it was inappropriate and unsafe to discharge into the environment.
pH in Synthetic Wastewater Samples
The nature of the synthetic wastewater prepared in this study is pH 5.55+0.49 (Table 2). After the treatment, the pH of the wastewater samples was drastically reduced upon adding H-EE and V-EE as they exhibited a lower pH range than the control sample’s pH (Table 3). This is due to the introduction of various organic acids from eco-enzyme in the fermented product, which lowers the pH of wastewater [25]. The pH of all eco-enzyme-treated samples did not undergo significant changes after 5 days, indicating that nitrification and other processes are still going on. Low pH in wastewater after adding EE will influence the decolourization of dye [43]. As Aris et al. (2007) suggested, limestone can be used to recover acidic water to water close to neutral [44].
Sodium Ions (Na+), Potassium Ions (K+), Calcium Ions (Ca2+), Nitrate (NO3 −), Ammonium (NH4 +), and Phosphate (PO4 3−) in Synthetic Wastewater Samples
Through anaerobic fermentation, NaCl (sodium chloride) concentration can influence the microbial community and enzyme activity [45]. The highest removal efficiency of Na+ was observed in samples under treatment VC (20%, v/v), which achieved a removal percentage of 13.5%, followed by treatment VB (10%, v/v) with 11.8% of Na+ removed after 5 days. After 5 days of treatment, Na+ in the synthetic wastewater is still above the Environment Protection Agency (EPA) (2003) [46] acceptable range as drinking water (20 mg/L). In this study, anaerobic fermentation was selected. Removal of sodium from wastewater up to 0.79 g Na/L requires aerobic fermentation by photosynthetic bacteria, Rhodovulum sp [47], within 8 days of treatment. This suggests that oxygen plays a key in removing Na+.
Microorganisms require cation for intracellular functions such as transportation systems [48]. Cation ions such as K+ and Ca2+ enhance biological phosphorus removal (EBPR) from wastewater [49]. EBPR is a wastewater treatment system based on the anaerobic and aerobic systems. Choi et al. (2011) [49] documented that potassium is soluble with phosphate in the anaerobic condition, and Ca2+ will not react to the process. The pH of the solution will influence Ca2+ removal. Low pH will inhibit calcium carbonate formation, and thus, efficiency in removing Ca2+ become lower. In the current study, VC shows the highest removal efficiency of K+ by 37.2% PO43− by 88.4%, respectively. Overall, all Ca2+ in this study is reduced compared to untreated synthetic wastewater (36.89 + 2.59 mg/L or ~3.7 mg/dL or 0.92 mmol/L).
This outcome might result from phosphate binding protein (PBP), which eliminates inorganic phosphate via the high-affinity adsorption process [50]. PBP is one of the phosphate-specific transporter complex components expressed by microbes like Pseudomonas sp. and Escherichia coli. These microbes are known as polyphosphate accumulating organisms (PAO) which had been identified in sewerage treatment plants as they are actively involved in enhanced biological phosphorus removal (EBPR) to reduce phosphorus load in sewerage [36, 51]. Thus, it was concluded that eco-enzymes could effectively reduce PO43− load in wastewater regardless of the fermentation period of the eco-enzyme, thereby improving the water quality and preventing the undesired incidence of eutrophication in water bodies upon the discharge of eco-enzyme-treated wastewater.
Denitrifying microorganisms produce nitrate reductase with the genera of Pseudomonas, Bacillus, Escherichia, Paraccocus, etc. Under anaerobic conditions, the oxygen from nitrate was used to oxidise carbon sources for energy utilisation. As a result, the nitrate level and the nitrogen content in wastewater are reduced [52]. The amount of NO3− directly connects with NH4+ through the nitrification process. In the natural environment, nitrogen cycling is associated with nitrate, ammonia, and ammonium cation, which involve a biological processes such as mineralisation and nitrification [53,54,55].
As shown in Tables 2 and 3, the concentration of NH4+ is reduced significantly after 5 days of treatment at different EE from 6.46 ± 0.55 mg/L to the range of 0.38 ± 0–0.98 ± 0 mg/L, respectively. It was observed that the concentration of NO3− in all treatments was increased from 217.78 ± 8.39 mg/L in synthetic wastewater to 5.43.61 ± 9.10 mg/L in HA. The presence of microbial consortium in the wastewater carries out the biological process to remove and convert NH4+ to NO3− [56, 57].
Total Dissolved Solids (TDS) of Synthetic Wastewater Samples
Total dissolved solids (TDS) measure the water samples’ organic matter and inorganic salts [58]. Moran (2018) mentioned that TDS above 500 mg/L is not suitable to use as drinking water [58]. Based on the findings, TDS from before treatment (705.67 ± 12.60 mg/L) and after 5 days of treatment was reduced in all treatments, including control (582.67 ± 5.55 mg/L), thus indicating that bacterial-based bioremediation was developed into nonsterile synthetic wastewater.
Dye Decolouration Assay
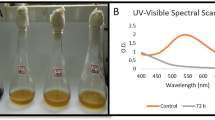
Dye in wastewater is one factor that correlates to water quality; hence, this study evaluated the capabilities of microbial consortium derived from fermented bio-waste in decolourising selected dyes. The initial and final concentrations of different types of dye in wastewater treated with fermented bio-waste were tabulated in Table 4, together with the decolourisation efficiency of each treatment. Moreover, statistical analysis of decolourisation efficiency between fermented bio-waste treatment and control treatment was also carried out by using a t-test.
Based on the data in Table 4, the decolourisation efficiency of dyes after bioremediation by different concentrations of H-EE and V-EE was determined. For crystal violet (CV), an overall reduction of dye concentration was observed in all samples. Of all samples, treatment VC exhibited the highest decolourisation efficiency of 13.53%. However, it does not have a significant difference with the control treatment as the statistical p-value was more than 0.05 and the remaining treatments. Hence, it was concluded that fermented bio-waste produced in this study is ineffective in decolourising CV. CV is an organic triphenylmethane dye with antimicrobial and antifungal properties. Due to its stable aromatic structure, CV is a recalcitrant with high resistance to environmental degradation [8]. However, ligninolytic enzymes such as laccases can decolourise CV via enzymatic degradation, which has been widely documented in many studies. Laccase is a multicopper oxidase that can degrade a range of synthetic dyes enzymatically, as it is not only involved in phenolic oxidations but also readily targets aromatic amines [7]. The electron-donating effect of methyl groups on CV’s nitrogen atoms is deemed more susceptible to oxidation reaction by laccase, thereby contributing to the decolourisation effect of CV by laccases [59]. Bharagava et al. (2018) [22] reported that CV could be degraded into benzene with less toxicity by laccases from Aeromonas hydrophilia, which have been isolated from textile wastewater. Effective enzymatic decolourisation of CV was further demonstrated in other studies when up to 97% of CV was decolourised by immobilised and free laccase extracted from Trametes modesta [59]. Therefore, compared to the findings reported by other researchers, the ineffectiveness of fruit or vegetable waste in CV decolourisation from this study might have resulted from the absence or insignificant catalytic activity of laccases in the extracted waste.
Interestingly, the HC treatment exhibited CV decolourisation efficiency of 2.73%, lower than the control treatment of 5.64%. CV with relatively lower photostability is prone to photodegradation upon exposure to light, especially via the photooxidation process. The formation of new species proved the photodegradation of CV at different wavelengths after illumination, which was indicated as the intermediates of CV [60]. However, Weyermann et al. (2009) [61] reported that singlet oxygen quenchers like β-carotene and zinc complexes could inhibit the photodecomposition of triphenylmethane dye like CV. Therefore, decolourisation of CV in control treatment was inferred by photodegradation during the handling process, such as spectrophotometric measurement, which happened under exposure to visible light and ultraviolet. Whilst the lower decolourisation efficiency in HC treatment might be due to the presence of compounds in H-EE that retarded the photodegradation of CV by reacting with singlet oxygen, thereby minimising the degradation of CV upon the attack by singlet oxygen. This inference was further enhanced by the decreasing CV decolourisation efficiency observed as the treatment concentration of H-EE increased.
In contrast, both H-EE and V-EE showed remarkable outcomes on methylene blue (MB) decolourisation, as up to 88.52% of MB was decolourised after 3 days under treatment HC. Besides the sample treated by VA, the remaining treatments had decolourisation efficiency, significantly different from the control treatment. Hence, the eco-enzyme produced in this study (from the fermented bio-waste) is known to have decolourisation efficacy on wastewater containing MB dye. Moreover, it was found that H-EE was more effective in removing MB than V-EE, as indicated by the differences in decolourisation efficiency between H-EE and V-EE (Table 4). The optimum concentration for MB decolourisation by H-EE and V-EE is 20% (v/v), indicating that the higher the concentration of fermented bio-waste, the more the MB will be decolourised.
In this study, the removal of MB from wastewater via bioremediation of H-EE and V-EE has potentially resulted from other ligninolytic enzymes, which have a degradative effect on a wide range of synthetic dyes. Lignin peroxidase is an oxidase with high redox potential compared to other ligninolytic enzymes like laccases and manganese peroxidase. Hence, thiazine dye like MB is more susceptible to biodegradation by lignin peroxidase. The enzymatic degradation of MB by lignin peroxidase was further proven by other studies where MB was utilised as the substrate for colourimetric assay to quantify lignin peroxidase activity [62]. Alam et al. (2009) [63] found lignin peroxidase produced by Phanerochaete chrysosporium, a white-rot fungus isolated from sewage sludge, effective in removing up to 40% of MB dye.
Furthermore, the result obtained in this study agreed with the findings, as around 82% of MB were successfully decolourised by the lignin peroxidase produced from P. chrysosporium [64]. Thus, eco-enzymes made in fermented bio-waste with significant decolourisation efficiency on MB dye are deemed to have lignin peroxidase, which actively degrades MB in the solution via oxidation and demethylation [65]. The pH and contact time are potential parameters that influence the effectiveness of the decolourization process. Both H-EE and V-EE show different pH. In addition, the decolourization also influences by the biomass if biosorbent is involved. Lastly, eco-enzyme could be a cost-effective tool in the bioremediation of textile wastewater containing a high concentration of dye if it includes other dye-degradative enzymes like azoreductase, manganese peroxidase, and microperoxidase-11 [39].
Conclusion
As for dye decolourisation, fermented bio-waste was ineffective in decolourising crystal violet but surprisingly exhibited substantial decolourisation efficiency on methylene blue. From here, the positive outcome of microbial consortium derived from fermented bio-waste on wastewater containing dyes shown in this study was inferred by the biochemical reactions catalysed or mediated by key enzymes and bioactive compounds in fermented bio-waste. Therefore, these compounds are the keys to their efficiency and are of utmost importance to be explored for in-depth discovery.
Data Availability
All relevant data are within the paper and its Supporting information file if any.
References
Ali A, Haseeb M (2019) Radiofrequency identification (RFID) technology as a strategic tool towards the higher performance of supply chain operations in textile and apparel industry of Malaysia. Uncertain Supply Chain Manag 7:215–226. https://doi.org/10.5267/j.uscm.2018.10.004
Rana RM, Neethu MK (2017) Degradation of methylene blue in textile waste using activated sawdust and eggshell biosorbent. Int J Adv Res Innov Ideas Educ 2(4):7–16
JM-Ridha M, Sahar IH, Ziad TA, Mohammed AA, Ghazi MA (2020) Biodegradation of reactive dyes by some bacteria using response surface methodology as an optimization technique. Alexandria Eng J 59(5):3551–3563. https://doi.org/10.1016/j.aej.2020.06.001
Wan Kamarudin WF, Abdul Rahman MN, Rahamat BN, Irwan Z, Yaafar MR (2022) Microbial degradation of food dye by Pseudomonas aeruginosa. AIP Conference Proceed 2454:050029. https://doi.org/10.1063/5.0078320
Goi CL (2020) The river water quality before and during the movement control order (MCO) in Malaysia. CSCEE 2:100027. https://doi.org/10.1016/j.cscee.2020.100027
Afroz R, Masud MM, Akhtar R, Duasa JB (2014) Water pollution: challenges and future direction for water resource management policies in Malaysia. Environ and urban ASIA 5(1):63–81
Moldes D, Fernández-Fernández M, Sanromán M (2012) Role of laccase and low molecular weight metabolites from trametes versicolor in dye decolorization. Sci World J 2012:1–9. https://doi.org/10.1100/2012/398725
Ali H, Shehata S, Ramadan K (2016) Microbial decolorization and degradation of crystal violet dye by Aspergillus niger. Int J Environ Sci Technol 13(12):2917–2926
Patel A, Patel V, Patel H, Trivedi U, Patel K (2020) White rot fungi: nature’s scavenger. In: Microbial Bioremediation & Biodegradation. Springer, pp 267–307
Li Y, Wang SF, Shen ZC, Li X, Zhou QY, Sun YX, Wang TT, Liu YF, Gao Q (2020) Gradient adsorption of methylene blue and crystal violet onto compound microporous silica from aqueous medium. ACS Omega 5(43):28382–28392. https://doi.org/10.1021/acsomega.0c04437
Abbasi B (2017) Removal of dye by biological methods using fungi. Int J Med Rev 4(4):112–118. https://doi.org/10.29252/ijmr-040405
Sarker MR, Chowdhury M, Deb AK (2019) Reduction of color intensity from textile dye wastewater using microorganisms: a review. INT J Curr Microbiol Appl Sci 8(2):3407–3415. https://doi.org/10.20546/ijcmas.2019.802.397
Alshehrei F (2020) Role of microorganisms in biodegradation of food additive Azo dyes: a review. Afr J Biotechnol 19(11):799–805. https://doi.org/10.5897/AJB2020.17250
Ahmed S, Aktar S, Zaman S, Jahan RA, Bari ML (2020) Use of natural bio-sorbent in removing dye, heavy metal and antibiotic-resistant bacteria from industrial wastewater. Appl Water Sci 10:107. https://doi.org/10.1007/s13201-020-01200-8
Pinheiro LRS, Gradíssimo DG, Xavier LP, Santos AV (2022) Degradation of azo dyes: bacterial potential for bioremediation. Sustainability 14:1510. https://doi.org/10.3390/su14031510
Arun C, Sivashanmugam P (2017) Study on optimization of process parameters for enhancing the multi-hydrolytic enzyme activity in garbage enzyme produced from pre-consumer organic waste. Biores Tech 226:200–210
Quilliam RS, Weidmann M, Moresco V, Purshouse H, O’Hara Z, Oliver DM (2020) COVID-19: the environmental implications of shedding SARS-CoV-2 in human faeces. Environ Int 140:105790
Department of Environment (DOE) (2009) Environmental quality (sewage & industrial effluent). Department of Environment, Putrajaya, Malaysia
Krom MD (1980) Spectrophotometric determination of ammonia: a study of a modified Berthelot reaction using salicylate and dichloroisocyanurate. Analyst 105(1249):305–316
Harris TM (2003) Analysis of sediments of zinc and phosphate ions. In: Henry DO (ed) Neanderthals in the Levant: Behavioural Organization and the Beginnings of Human Modernity. A&C Black, London, pp 288–195
Rakhmawatie MD, Wibawa T, Lisdiyanti P, Pratiwi WR (2019) Evaluation of crystal violet decolorization assay and resazurin microplate assay for antimycobacterial screening. Heliyon 5(8):e02263. https://doi.org/10.1016/j.heliyon.2019.e02263
Bharagava RN, Mani S, Mulla SI, Saratale GD (2018) Degradation and decolourization potential of a ligninolytic enzyme producing Aeromonas hydrophila for crystal violet dye and its phytotoxicity evaluation. Ecotoxicol Environ Saf 156:166–175
Franca RDG, Vieira A, Carvalho G, Oehmen A, Pinheiro HM, Crespo MTB, Lourenço ND (2020) Oerskovia paurometabola can efficiently decolorize azo dye Acid Red 14 and remove its recalcitrant metabolite. Ecotoxicol Environ Saf 191:110007. https://doi.org/10.1016/j.ecoenv.2019.110007
Harmen S, Faridah Y, Md Zahangir A (2021) A new technique to estimate percentage decolorization of synthetic dyes on solid media by extracellular laccase from white-rot fungus. Bioremediation J 2021:1–9. https://doi.org/10.1080/10889868.2021.1984197
Rasit N, Hwe Fern L, Ab Karim G, Wan Azlina W (2019) Production and characterization of eco enzyme produced from tomato and orange wastes and its influence on the aquaculture sludge. Int J Innov Res Technol Sci Eng And Tech 10(3):967–980
Arun C, Sivashanmugam P (2015a) Identification and optimization of parameters for the semi-continuous production of garbage enzyme from pre-consumer organic waste by green RP-HPLC method. Waste manag 44:28–33
Nazim F, Meera V (2017) Comparison of treatment of greywater using garbage and citrus enzymes. Int J Innov Res Technol Sci Eng And Tech 6:4. http://www.ijirset.com/upload/2017/nctacme/9_12_NCTACME_17%20CE%20005-02.pdf
Hadj Saadoun J, Bertani G, Levante A, Vezzosi F, Ricci A, Bernini V, Lazzi C (2021) Fermentation of agri-food waste: a promising route for the production of aroma compounds. Foods (Basel, Switzerland) 10(4):707. https://doi.org/10.3390/foods10040707
Czech A, Zarycka E, Yanovych D, Zasadna Z, Grzegorczyk I, Kłys S (2020) Mineral content of the pulp and peel of various citrus fruit cultivars. Biol Trace Elem Res 193(2):555–563
Sharma KD, Karki S, Thakur NS, Attri S (2012) Chemical composition, functional properties and processing of carrot—a review. J Food Sci Technol 49(1):22–32
Feyera M, Abera S, Temesgen M (2020) Effect of fermentation time and blending ratio on nutrients and some antinutrient composition of coplenetary flour. J Food Nutr Res 5(1):118–130
Arun C, Sivashanmugam P (2015b) Solubilization of waste-activated sludge using a garbage enzyme produced from different pre-consumer organic waste. RSC adv 5(63):51421–51427
Ademollo N, Patrolecco L, Polesello S, Valsecchi S, Wollgast J, Mariani G, Hanke G (2012) The analytical problem of measuring total concentrations of organic pollutants in whole water. TrAC, Trends Anal Chem 36:71–81
Bernhard A (2010) The nitrogen cycle: processes, players, and human. Nat Sci Educ 2(2):12
Oehmen A, Yuan Z, Blackall LL, Keller J (2005) Comparison of acetate and propionate uptake by polyphosphate accumulating organisms and glycogen accumulating organisms. Biotechnol Bioeng 91(2):162–168
Bunce JT, Ndam E, Ofiteru ID, Moore A, Graham DW (2018) A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front Environ Sci 6:8
Nazim F, Meera V (2013) Treatment of synthetic greywater using 5% and 10% garbage enzyme solution. bonfring int. J. Ind. Eng and manag sci 3(4):111–117
Sambaraju S, Lakshmi VS (2020) Eco-friendly treatment of dairy wastewater using garbage enzyme. Mater Today: Proceedings 33:650–653
Tang FE, Tong CW (2011) A study of the garbage enzyme’s effects in domestic wastewater. World Acad Sci Eng Technol 60:1146–1148
Zhao Y, Zhuang X, Ahmad S, Sung S, Ni SQ (2020) Biotreatment of high-salinity wastewater: current methods and future directions. World J Microbiol Biotechnol 36(3):1–11
Moss B (2008) Water pollution by agriculture. Philos Trans R Soc B: Biological Sciences 363(1491):659–666
Meiramkulova K, Mkilima T, Baituk G, Beisembayeva K, Meirbekov A, Kakabayev A, Adilbektegi G, Tleukulov A, Tazhkenova G (2022) Treatment of waste stabilization pond effluent using natural zeolite for irrigation potential. PloS one 17(6):e0259614. https://doi.org/10.1371/journal.pone.0259614
Yaseen DA, Scholz M (2019) Impact of pH on treating artificial textile wastewater containing azo dyes using pond systems. Int. J Environ Res 13:367–385. https://doi.org/10.1007/s41742-019-00180-1
Aris A, Mokhtar N, Muslim R, Ujang Z, Majid Z (2007) Treatment of acidic water from Bekok River using open limestone channel. Water Supply 7(4):65–71. https://doi.org/10.2166/ws.2007.145
Pang H, Xin X, He J, Cui B, Gup D, Liu S, Yan Z, Liu C, Wang X, Nan J (2020) Effect of NaCl concentration on microbiological properties in NaCl assistant anaerobic fermentation: hydrolase activity and microbial community distribution. Front Microbiol 11:589222. https://doi.org/10.3389/fmicb.2020.589222
Environmental Protection Agency (2003). Drinking water advisory: consumer acceptability advice and health effects analysis on sodium. www.epa.gov/safewater/ccl/pdf/sodium.pdf
Sasaki K, Hosokawa Y, Taken K, Sasaki K (2017) Removal of sodium from seawater medium using photosynthetic bacteria. J Agric Food Chem 6:3. https://doi.org/10.4236/jacen.2017.63008
Epstein W (2003) The roles and regulation of potassium in bacteria. Prog Nucleic Acid Res Mol Biol 75:293–320. https://doi.org/10.1016/s0079-6603(03)75008-9
Choi HJ, Yu SW, Lee SM, Yu SY (2011) Effects of potassium and magnesium in the enhanced biological phosphorus removal process using a membrane bioreactor. Water Environ Res 83(7):613–621. http://www.jstor.org/stable/24239887
Venkiteshwaran K, Pokhre N, Hussein F, Antony E, Mayer BK (2018) Phosphate removal and recovery using immobilized phosphate binding proteins. Water Res X 1:100003. https://doi.org/10.1016/j.wroa.2018.09.003
Li Q, Yu Z, Shao X, He J, Li L (2009) Improved phosphate biosorption by bacterial surface display of phosphate-binding protein utilizing ice nucleation protein. FEMS Microbiol Lett 299(1):44–52
Ni BJ, Pan YT, Guo JH, Virdis B, Hu SH, Chen XM, Yuan ZG (2016) Denitrification processes for wastewater treatment. Metalloenzymes in Denitrification, pp 368–418. https://doi.org/10.1039/9781782623762-00368
Zhang T, Ye L, Tong AHY, Shao MF, Lok S (2011) Ammonia-oxidizing archaea and ammonia-oxidizing bacteria in six full-scale wastewater treatment bioreactors. Appl Microbiol Biotechnol 91(4):1215–1225
Lájer K (2012) Ammonium removal by nitrification in drinking water treatment. Kvalitet voda 10:47–53
Lehtovirta-Morley LE (2018) Ammonia oxidation: ecology, physiology, biochemistry and why they must all come together. FEMS Microbiol Lett 365(9):fny058
Mohanty SR, Nagarjuna M, Parmar R, Ahirwar U, Patra A, Dubey G, Kollah B (2019) Nitrification rates are affected by biogenic nitrate and volatile organic compounds in agricultural soils. Front Microbiol 10:772
Rajta A, Bhatia R, Setia H, Pathania P (2020) Role of heterotrophic aerobic denitrifying bacteria in nitrate removal from wastewater. J Appl Microbiol 128(5):1261–1278
Moran S (2018) An applied guide to water and effluent treatment plant design. Elsevier. https://doi.org/10.1016/C2016-0-01092-6
Kandelbauer A, Maute O, Kessler RW, Erlacher A, Gübitz GM (2004) Study of dye decolorization in an immobilised laccase enzyme-reactor using online spectroscopy. Biotechnol Bioeng 87(4):552–563
Zakharian TY (2000) Photodecomposition of crystal violet dye in water solution and suspensions of metal oxides. The University of Northern Iowa, Thesis (Master of Arts)
Weyermann C, Kirsch D, Costa Vera C, Spengler B (2009) Evaluation of the photodegradation of crystal violet upon light exposure by mass spectrometric and spectroscopic methods. J of forensic sci 54(2):339–345
Magalhães DB, de Carvalho MEA, Bon E, Neto JSA, Kling SH (1996) Colourimetric assay for lignin peroxidase activity determination using methylene blue as substrate. Biotechn techniques 10(4):273–276
Alam MZ, Mansor MF, Jalal K (2009) Optimization of decolorization of methylene blue by lignin peroxidase enzyme produced from sewage sludge with Phanerocheate chrysosporium. J Hazard Mater 162(2-3):708–715
Chai CC (2009) Enhanced production of lignin peroxidase and manganese peroxidase by phanerochaete chrysosporium in a submerged culture fermentation and their application in decolourisation of dyes. Dissertation (Master of Science). Universiti Sains Malaysia, Malaysia
Ferreira VS, Magalhães DB, Kling SH, Da Silva JG, Bon EP (2000) N-demethylation of methylene blue by lignin peroxidase from phanerochaete chrysosporium. In: twenty-first symposium on biotechnology for fuels and chemicals, Totowa, NJ, Springer, pp 255–265
Acknowledgements
The corresponding author is thankful to all co-authors for their technical guidance in completing the present work and preparing the manuscript. This work is supported by Research Excellence & Innovation Grant (REIF-FAS-2021/036), UCSI University, Malaysia.
Funding
This work is supported by Research Excellence & Innovation Grant (REIF-FAS-2021/036), UCSI University, Malaysia.
Author information
Authors and Affiliations
Contributions
Regina Leong Zhi Ling: manuscript preparation and post-data analysis.
Tee Jia Jian: conducting experiment and pre-data analysis.
Lim Lai Huat: produce fermented product, manuscript edited and response to reviewer comments.
Teo Swee Sen: data evaluation and project supervisor.
Corresponding author
Ethics declarations
Ethical Approval
No ethical application requirements for this project.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leong, R.Z., Tee, J.J., Lim, L. et al. Decolourization of Crystal Violate and Methylene Blue Wastewater Using Anaerobic Fermented Bio-Waste. Water Conserv Sci Eng 8, 15 (2023). https://doi.org/10.1007/s41101-023-00189-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41101-023-00189-w