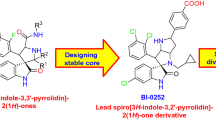
Abstract
As a result of the toxicity of currently available anticancer drugs and the inefficiency of chemotherapeutic treatments, the design and discovery of effective and selective antitumor agents continues to be a hot topic in organic medicinal chemistry. Targeted therapy is a newer type of cancer treatment that uses drugs designed to interfere with specific molecules necessary for tumor growth and progression. This review explains the mechanism of regulation of p53 (tumor suppressor protein) by MDM2 and illustrates the role of targeting p53–MDM2 protein–protein interaction using small molecules as a new cancer therapeutic strategy. Spirocyclic oxindoles or spiro-oxindoles, with a rigid heterocyclic ring fused at the 3-position of the oxindole core with varied substitution around it, are the most efficacious class of small molecules which inhibit cell proliferation and induce apoptosis in cancer cells, leading to complete tumor growth regression without affecting activities of normal cells. In this review, we present a comprehensive account of the systematic development of and recent progress in diverse spiro-oxindole derivatives active as potent selective inhibitors of p53–MDM2 interaction with special emphasis on spiro-pyrrolidinyl oxindoles (the MI series), their mechanism of action, and structure–activity relationship. This review will help in understanding the molecular mechanism of p53 reactivation by spiro-oxindoles in tumor tissues and also facilitates the design and exploration of more potent analogues with high efficacy and low side effects for the treatment of cancer.
Graphical Abstract
Recent progress in spiro-oxindole derivatives as potent small molecule inhibitors of p53–MDM2 interaction, useful as anticancer agents, is described with reference to their mechanism of action and structure–activity relationship.









Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) CA Cancer J Clin 65:87–108
WHO (2014) World cancer report, Chap 1.1. WHO, Geneva
Chabner BA, Roberts TG Jr (2005) Nat Rev Cancer 5:65–72
Lind MJ (2011) Principles of cytotoxic chemotherapy. Medicine 39(12):711–716
Rheingold SR, Neugut AI, Meadows AT (2003) Secondary cancers: incidence, risk factors, and management. Chapter 159. In: Kufe DW, Pollock RE, Weichselbaum RR et al (eds) Holland-Frei cancer medicine, 6th edn. BC Decker, Hamilton
Schreiber SL (2003) Chem Eng News 81:51–61
Bae YH, Mrsny RJ, Park K (2013) Cancer targeted drug delivery: an elusive dream. Springer, Berlin
Aggarwal S (2010) Nat Rev Drug Discov 9:427–428
Chessum N, Jones K, Pasqua E, Tucker M (2015) Prog Med Chem 54:1–63
Stratton MR (2011) Science 331:1553–1558
Collins I, Workman P (2006) Nat Chem Biol 2:689–700
Kummar S, Murgo AJ, Tomasze JE, Doroshow JH (2014) Therapeutic targeting of cancer cells: era of molecularly targeted agents. Elsevier Churchill Livingstone, Philadelphia
Gerber DE (2008) Am Fam Physician 77:311–319
Lacroix M (2014) Targeted therapies in cancer. Nova Sciences, Hauppauge. ISBN 978-1-63321-687-7
Tanner JE (2005) Cancer Metastasis Rev 24:585–598
Avendano C, Menendez JC (2015) Medicinal chemistry of anticancer drugs, 2nd edn. Elsevier, Amsterdam
Zhao Y, Bernard D, Wang S (2013) BioDiscovery 8(4):1–15
Dickens MP, Fitzgerald R, Fischer PM (2010) Semin Cancer Biol 20:10–18
Zhao Y, Aguilar A, Bernard D, Wang S (2015) J Med Chem 58:1038–1052
Vanneman M, Dranoff G (2012) Nat Rev Cancer 12:237–251
Masters GA, Krilov L, Bailey HH, Brose MS, Burstein H, Diller LR, Dizon DS, Fine HA, Kalemkerian GP, Moasser M, Neuss MN, O’Day SJ, Odenike O, Ryan CJ, Schilsky RL, Schwartz GK, Venook AP, Wong SL, Patel JD (2015) J Clin Oncol 33:786–809
Abramson R (2015) Overview of targeted therapies for cancer. My Cancer Genome. https://www.mycancergenome.org/content/molecular-medicine/overview-of-targeted-therapies-for-cancer/. Accessed 1 Jan 2016
Mullard A (2015) Nat Rev Drug Discov 14:77–81
Wang S, Zhao Y, Bernard D, Aguilar A, Kumar S (2012) Top Med Chem 8:57–80
Lane DP, Cheok CF, Lain S (2010) Cold Spring Harb Perspect Biol 2:a001222
Vogelstein B, Lane D, Levine AJ (2000) Nature 408:307–310
Moll UM, Petrenko O (2003) Mol Cancer Res 1:1001–1008
Fu T, Min H, Xu Y, Chen J, Li G (2012) Int J Mol Sci 13:9709–9740
Vu BT, Vassilev L (2011) Curr Top Microbiol Immunol 348:151–172
Picksley SM, Lane DP (1993) BioEssays 15:689–690
Wade M, Wang YV, Wahl GM (2010) Trends Cell Biol 20:299–309
Momand J, Wu HH, Dasgupta G (2000) Gene 242:15–29
Hainaut P, Hollstein M (2000) Adv Cancer Res 77:81–137
Vousden KH, Lu X (2002) Nat Rev Cancer 2:594–604
Riedinger C, McDonnell JM (2009) Future Med Chem 1:1075–1094
Shangary S, Wang S (2009) Annu Rev Pharmacol Toxicol 49:223–241
Chene P (2003) Nat Rev Cancer 3:102–109
Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, Nikolovska-Coleska Z, Ding K, Wang G, Chen J, Bernard D, Zhang J, Lu Y, Gu Q, Shah RB, Pienta KJ, Ling X, Kang S, Guo M, Sun Y, Yang D, Wang S (2008) Proc Natl Acad Sci USA 105:3933–3938
Zhang R, Wang H, Agrawal S (2005) Curr Cancer Drug Targets 5:43–49
Herman AG, Hayano M, Poyurovsky MV, Shimada K, Skouta R, Prives C, Stockwell BR (2011) Cancer Discov 1:312–325
Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, Van Pelt CS, Lozano G (2008) Genes Dev 22:1337–1344
Hoelder S, Clarke PA, Workman P (2012) Mol Oncol 6:155–176
Azmi AS, Beck FW, Sarkar FH, Mohammad RM (2011) Curr Pharm Des 17:640–652
Zheng T, Wang J, Song X, Meng X, Pan S, Jiang H, Liu L (2010) J Cancer Res Clin Oncol 136:1597–1604
Wade M, Li YC, Wahl GM (2013) Nat Rev Cancer 13:83–96
Millard M, Pathania D, Grande F, Xu S, Neamati N (2011) Curr Pharm Des 17:536–559
Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP (1996) Science 274:948–953
Vazquez A, Bond EE, Levine AJ, Bond GL (2008) Nat Rev Drug Discov 7:979–987
Pujals A, Favre L, Gaulard P, Wiels J (2015) Activation of wild-type p53 by MDM2 inhibitors: a new strategy for lymphoma treatment. Blood Lymphat Cancer 5:93–100
Khoo KH, Verma CS, Lane DP (2014) Nat Rev Drug Discov 13:217–236
Wade M, Wahl GM (2009) Mol Cancer Res 7:1–11
Qin JJ, Wang W, Voruganti S, Wang H, Zhang WD, Zhang R (2015) Oncotarget 6:2623–2640
Qin JJ, Nag S, Voruganti S, Wang W, Zhang R (2012) Curr Med Chem 19:5705–5725
Bottger V, Bottger A, Howard SF, Picksley SM, Chene P, Garcia-Echeverria C, Hochkeppel HK, Lane DP (1996) Oncogene 13:2141–2147
Kritzer JA, Lear JD, Hodsdon ME, Schepartz A (2004) J Am Chem Soc 126:9468–9469
Zhang Q, Zeng SX, Lu H (2014) Subcell Biochem 85:281–319
Aeluri M, Chamakuri S, Dasari B, Guduru SK, Jimmidi R, Jogula S, Arya P (2014) Chem Rev 114:4640–4649
Carry JC, Garcia-Echeverria C (2013) Bioorg Med Chem Lett 23:2480–2485
Vassilev Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA (2004) Science 303:844–848
Tovar C, Graves B, Packman K, Filipovic Z, Higgins B, Xia M, Tardell C, Garrido R, Lee E, Kolinsky K, To KH, Linn M, Podlaski F, Wovkulich P, Vu B, Vassilev LT (2013) Cancer Res 73:2587–2597
Ray-Coquard I, Blay JY, Italiano A, Le CA, Penel N, Zhi J, Heil F, Rueger R, Graves B, Ding M, Geho D, Middleton SA, Vassilev LT, Nichols GL, Bui BN (2012) Lancet Oncol 13:1133–1140
Rew Y, Sun D, De Gonzalez-Lopez TF, Bartberger MD, Beck HP, Canon J, Chen A, Chow D, Deignan J, Fox BM, Gustin D, Huang X, Jiang M, Jiao X, Jin L, Kayser F, Kopecky DJ, Li Y, Lo MC, Long AM, Michelsen K, Oliner JD, Osgood T, Ragains M, Saiki AY, Schneider S, Toteva M, Yakowec P, Yan X, Ye Q, Yu D, Zhao X, Zhou J, Medina JC, Olson SH (2012) J Med Chem 55:4936
Sun D, Li Z, Rew Y, Gribble M, Bartberger MD, Beck HP, Canon J, Chen A, Chen X, Chow D, Deignan J, Duquette J, Eksterowicz J, Fisher B, Fox BM, Fu J, Gonzalez AZ, De Gonzalez-Lopez TF, Houze JB, Huang X, Jiang M, Jin L, Kayser F, Liu JJ, Lo MC, Long AM, Lucas B, McGee LR, McIntosh J, Mihalic J, Oliner JD, Osgood T, Peterson ML, Roveto P, Saiki AY, Shaffer P, Toteva M, Wang Y, Wang YC, Wortman S, Yakowec P, Yan X, Ye Q, Yu D, Yu M, Zhao X, Zhou J, Zhu J, Olson SH, Medina JC (2014) J Med Chem 57:1454–1472
Gonzalez AZ, Li Z, Beck HP, Canon J, Chen A, Chow D, Duquette J, Eksterowicz J, Fox BM, Fu J, Huang X, Houze J, Jin L, Li Y, Ling Y, Lo MC, Long AM, McGee LR, McIntosh J, Oliner JD, Osgood T, Rew Y, Saiki AY, Shaffer P, Wortman S, Yakowec P, Yan X, Ye Q, Yu D, Zhao X, Zhou J, Olson SH, Sun D, Medina JC (2014) J Med Chem 57:2963–2988
Canon J, Osgood T, Olson SH, Saiki AY, Robertson R, Yu D, Eksterowicz J, Ye Q, Jin L, Chen A, Zhou J, Cordover D, Kaufman S, Kendall R, Oliner JD, Coxon A, Radinsky R (2015) Mol Cancer Ther 14:649–658
Raboisson P, Marugan JJ, Schubert C, Koblish HK, Lu T, Zhao S, Player MR, Maroney AC, Reed RL, Huebert ND, Lattanze J, Parks DJ, Cummings MD (2005) Bioorg Med Chem Lett 15:1857–1861
Marugan JJ, Leonard K, Raboisson P, Gushue JM, Calvo R, Koblish HK, Lattanze J, Zhao S, Cummings MD, Player MR, Schubert C, Maroney AC, Lu T (2006) Bioorg Med Chem Lett 16:3115–3120
Allen JG, Bourbeau MP, Wohlhieter GE, Bartberger MD, Michelsen K, Hungate R, Gadwood RC, Gaston RD, Evans B, Mann LW, Matison ME, Schneider S, Huang X, Yu D, Andrews PS, Reichelt A, Long AM, Yakowec P, Yang EY, Lee TA, Oliner JD (2009) J Med Chem 52:7044–7053
Beck HP, DeGraffenreid M, Fox B, Allen JG, Rew Y, Schneider S, Saiki AY, Yu D, Oliner JD, Salyers K, Ye Q, Olson S (2011) Bioorg Med Chem Lett 21:2752–2755
Yin H, Lee GI, Park HS, Payne GA, Rodriguez JM, Sebti SM, Hamilton AD (2005) Angew Chem Int Ed Engl 44:2704–2707
Hardcastle IR, Ahmed SU, Atkins H, Farnie G, Golding BT, Griffin RJ, Guyenne S, Hutton C, Kallblad P, Kemp SJ, Kitching MS, Newell DR, Norbedo S, Northen JS, Reid RJ, Saravanan K, Willems HM, Lunec J (2006) J Med Chem 49:6209–6221
Ding Q, Zhang Z, Liu JJ, Jiang N, Zhang J, Ross TM, Chu XJ, Bartkovitz D, Podlaski F, Janson C, Tovar C, Filipovic ZM, Higgins B, Glenn K, Packman K, Vassilev LT, Graves B (2013) J Med Chem 56:5979–5983
Ma Y, Lahue BR, Shipps GW Jr, Brookes J, Wang Y (2014) Bioorg Med Chem Lett 24:1026–1030
Zak K, Pecak A, Rys B, Wladyka B, Domling A, Weber L, Holak TA, Dubin G (2013) Expert Opin Ther Pat 23:425–448
Ding K, Lu Y, Nikolovska-Coleska Z, Qiu S, Ding Y, Gao W, Stuckey J, Krajewski K, Roller PP, Tomita Y, Parrish DA, Deschamps JR, Wang S (2005) J Am Chem Soc 127:10130–10131
Chen L, Han X, Yang S, Zhang Z (2012) 3,3′-Spiroindolinone derivatives and their use for cancer. Patent No. EP2421866A1
Wang S, Sun W, Zhao Y, McEachern D, Meaux I, Barriere C, Stuckey JA, Meagher JL, Bai L, Liu L, Hoffman-Luca CG, Lu J, Shangary S, Yu S, Bernard D, Aguilar A, Dos-Santos O, Besret L, Guerif S, Pannier P, Gorge-Bernat D, Debussche L (2014) Cancer Res 74:5855–5865
Ding K, Lu Y, Nikolovska-Coleska Z, Wang G, Qiu S, Shangary S, Gao W, Qin D, Stuckey J, Krajewski K, Roller PP, Wang S (2006) J Med Chem 49:3432–3435
Yu B, Yu DQ, Liu HM (2015) Eur J Med Chem 97:673–698
Weber L (2010) Expert Opin Ther Pat 20:179–191
Kamal A, Mohammed AA, Shaik TB (2012) Expert Opin Ther Pat 22:95–105
Czarna A, Popowicz GM, Pecak A, Wolf S, Dubin G, Holak TA (2009) Cell Cycle 8:1176–1184
Hu B, Gilkes DM, Farooqi B, Sebti SM, Chen J (2006) J Biol Chem 281:33030–33035
Ding K, Wang G, Deschamps JR, Parrish DA, Wang S (2005) Tetrahedron Lett 46:5949–5951
Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD (2003) Proteins 52:609–623
Wang S, Zhao Y, Sun W, Kumar S, Leopold L, Debussche L, Barriere C, Carry J-C, Amaning K (2014) Spiro-oxindole MDM2 antagonists. US 14/170,101 (US20140148494A1)
Shangary S, Ding K, Qiu S, Nikolovska-Coleska Z, Bauer JA, Liu M, Wang G, Lu Y, McEachern D, Bernard D, Bradford CR, Carey TE, Wang S (2008) Mol Cancer Ther 7:1533–1542
Yu S, Qin D, Shangary S, Chen J, Wang G, Ding K, McEachern D, Qiu S, Nikolovska-Coleska Z, Miller R, Kang S, Yang D, Wang S (2009) J Med Chem 52:7970–7973
Huang W, Cai L, Chen C, Xie X, Zhao Q, Zhao X, Zhou HY, Han B, Peng C (2016) J Biomol Struct Dyn 34:341–351
Aguilar A, Sun W, Liu L, Lu J, McEachern D, Bernard D, Deschamps JR, Wang S (2014) J Med Chem 57:10486–10498
Ball-Jones NR, Badillo JJ, Franz AK (2012) Org Biomol Chem 10:5165–5181
Jones G, Willett P, Glen RC, Leach AR, Taylor R (1997) J Mol Biol 267:727–748
Popowicz GM, Czarna A, Wolf S, Wang K, Wang W, Domling A, Holak TA (2010) Cell Cycle 9:1104–1111
Khan A, Lu H (2008) Cancer Biol Ther 7:853–855
Sun SH, Zheng M, Ding K, Wang S, Sun Y (2008) Cancer Biol Ther 7:845–852
Azmi AS, Aboukameel A, Banerjee S, Wang Z, Mohammad M, Wu J, Wang S, Yang D, Philip PA, Sarkar FH, Mohammad RM (2010) Eur J Cancer 46:1122–1131
Azmi AS, Philip PA, Aboukameel A, Wang Z, Banerjee S, Zafar SF, Goustin AS, Almhanna K, Yang D, Sarkar FH, Mohammad RM (2010) Curr Cancer Drug Targets 10:319–331
Mohammad RM, Wu J, Azmi AS, Aboukameel A, Sosin A, Wu S, Yang D, Wang S, Al-Katib AM (2009) Mol Cancer 8:115
Zhao Y, Yu S, Sun W, Liu L, Lu J, McEachern D, Shargary S, Bernard D, Li X, Zhao T, Zou P, Sun D, Wang S (2013) J Med Chem 56:5553–5561
Wang S, Sun W, Yu S et al (2011) Highly potent and optimized small-molecule inhibitors of MDM2 achieve complete tumor regression in animal models of solid tumors and leukemia. Abstract LB-204. AACR 102nd annual meeting, Orlando, FL
Bill KL, Garnett J, Meaux I, Ma X, Creighton CJ, Bolshakov S, Barriere C, Debussche L, Lazar AJ, Prudner BC, Casadei L, Braggio D, Lopez G, Zewdu A, Bid H, Lev D, Pollock RE (2016) Clin Cancer Res 22:1150–1160
Chen L, Ding Q, Liu J, Yang S, Zhang Z, Hoffmann-La RIU (2009) Spiroindolinone derivatives. US7495007 B2
Chen L, Han X, Yang S, Zhang Z (2010) Preparation of 3,3′-spiroindolinones for treatment of cancer. WO 2010121995 A1
Chen L, Han X, He Y, Yang S, Zhang Z. Hoffman-La Roche Inc, USA (2009) Spiroindolinone derivatives as interaction inhibitors between p53 and MDM2 proteins and their preparation, pharmaceutical compositions and use in the treatment of cancer. US 20090163512 A1
Ding Q, Liu JJ, Zhang Z. Zhang Z, Hoffmann La Roche, Switzerland (2007) Spiroindolinone derivatives. WO 2007104714 A1
Chen L, Ding Q, Liu JJ, Yang S, Zhang Z (2007) Preparation of spiroindolinone derivatives as antitumor agents. US 20070213341 A1
Ding Q, Jiang N, Yang S, Zhang J, Zhang Z (2009) Spiroindolinone derivatives. US 20090156610 A1
Liu JJ, Zhang Z. (2009) Spiroindolinone derivatives. US 7638548 B2
Chen L, Han X, Yang S, Zhang Z (2010) Spiroindolinone pyridine derivatives as inhibitors of MDM2–p53 protein interaction useful as potent and selective anticancer agents and preparation thereof. US 20100204257
Zhang J, Zhang Z Spiroindolinone pyridine derivatives as inhibitors of MDM2–p53 protein interaction useful as potent and selective anticancer agents and preparation thereof. US 20100210674
Liu J-J, Zhang Z, Hoffmann-La Roche (2008) Preparation of spiroindole pyridotriazinediones as anticancer drugs. WO 2008141975
Bertamino A, Soprano M, Musella S, Rusciano MR, Sala M, Vernieri E, Di Sarno V, Limatola A, Carotenuto A, Cosconati S, Grieco P, Novellino E, Illario M, Campiglia P, Gomez-Monterrey I (2013) J Med Chem 56:5407–5421
Gomez-Monterrey I, Bertamino A, Porta A, Carotenuto A, Musella S, Aquino C, Granata I, Sala M, Brancaccio D, Picone D, Ercole C, Stiuso P, Campiglia P, Grieco P, Ianelli P, Maresca B, Novellino E (2010) J Med Chem 53:8319–8329
Ribeiro CJ, Amaral JD, Rodrigues CM, Moreira R, Santos MM (2014) Bioorg Med Chem 22:577–584
Liu J-J, Tilley JW, Zhang Z (2008) 3,3-Spiroindolinone derivatives. US 12/101182 US20080287421 A1
Acknowledgements
AKG acknowledges the Department of Science and Technology (DST, New Delhi) for financial support under the Women Scientist Project Scheme (WOS-A).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Alpana K. Gupta, Mausumi Bharadwaj, Anoop Kumar, and Ravi Mehrotra declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gupta, A.K., Bharadwaj, M., Kumar, A. et al. Spiro-oxindoles as a Promising Class of Small Molecule Inhibitors of p53–MDM2 Interaction Useful in Targeted Cancer Therapy. Top Curr Chem (Z) 375, 3 (2017). https://doi.org/10.1007/s41061-016-0089-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-016-0089-0