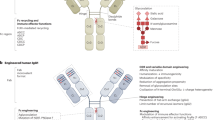
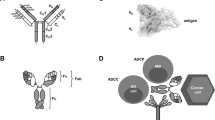
Summary
The past five years have witnessed the emergence of monoclonal antibodies as important therapeutics for cancer treatment. Lower toxicity for antibodies versus small molecules, the potential for increased efficacy by conjugation to radioisotopes and cellular toxins, or the ability to exploit immune cell functions have led to clinical performances on par or superior to conventional drug therapies. This review outlines the various immunoglobulin design strategies currently available, techniques used to reduce Ig antigenicity and toxicity, and points to consider during the manufacture of antibodies for use in clinical oncology.
Similar content being viewed by others
References
Mehren MM, Adams GP, Weiner LM: Monoclonal antibody therapy for cancer. Annu Rev Med 54: 343–369, 2003
Ross J, Gray K, Schenkein D, Greene B, Gray GS, Shulok J, Worland PJ, Celniker A, Rolfe M: Antibody-based therapeutics in oncology. Expert Rev Anticancer Ther3: 107–121, 2003
Glennie MJ, van de Winkel JG: Renaissance of cancer therapeutic antibodies.Drug Discov Today 8: 503–510, 2003
Riechmann L, Clark M, Waldmann H, Winter G: Reshaping human antibodies fortherapy. Nature 332: 323–327, 1988
Gilliland LK, Walsh LA, Frewin MR, Wise MP, Tone M, Hale G, Kioussis D, Waldmann H: Elimination of the immunogenicity of therapeutic antibodies. J Immunol 162:3663–3671, 1999
Roguska MA, Pedersen JT, Keddy CA, Henry AH, Searle SJ, Lambert JM, Goldmacher VS, Blattler WA, Rees AR, Guild BC: Humanization of murine monoclonalantibodies through variable domain resurfacing. Proc Natl Acad Sci USA 91: 969–973, 1994
Kuus-Reichel K, Grauer LS, Karavodin LM, Knott C, Krusemeier M, Kay NE: Willimmunogenicity limit the use, efficacy, and future development of therapeutic monoclonalantibodies? Clin Diagn Lab Immunol 1: 365–372, 1994
Pimm MV: Possible consequences of human antibody responses on thebiodistribution of fragments of human, humanized or chimeric monoclonal antibodies: A noteof caution. Life Sci 55: L45–L49, 1994
Feldman M, Maini RN: Anti-TNF alphatherapy or rheumatoid arthritis: Whathave we learned? Ann Rev Immunol 19: 163–196, 2001
Moreland LW, Heck LW, Jr, Koopman WJ: Biologic agents for treatingrheumatoid arthritis. Concepts and progress. Arthritis Rheum 40: 397–409, 1997
Kavanaugh AF: Anti-tumor necrosis factor-alpha monoclonal antibody therapyfor rheumatoid arthritis. Rheum Dis Clin North Am 24: 593–614, 1998
Legouffe E, Liautard J, Gaillard JP, Rossi JF, Wijdenes J, Bataille R, Klein B, Brochier J: Human anti-mouse antibody response to the injection of murine monoclonalantibodies against IL-6. Clin Exp Immunol 98: 323–329, 1994
Garrison L, McDonnell ND: Etanercept: Therapeutic use in patients withrheumatoid arthritis. Ann Rheum Dis 58(Suppl 1): I65–I69, 1999
Maeda T, Yamada Y, Tawara M, Yamasaki R, Yakata Y, Tsutsumi C, Onimaru Y, Kamihira S, Tomonaga M: Successful treatment with a chimeric anti-CD20 monoclonal antibody(IDEC-C2B8, rituximab) for a patient with relapsed mantle cell lymphoma who developed ahuman anti-chimeric antibody. Int J Hematol 74: 70–75, 2001
Tcheng JE, Kereiakes DJ, Lincoff AM, George BS, Kleiman NS, Sane DC, Cines DB, Jordan RE, Mascelli MA, Langrall MA, Damaraju L, Schantz A, Effron MB, Braden GA:Abciximab readministration: Results of the Reo Pro Readministration Registry. Circulation104: 870–875, 2001
Piro LD, White CA, Grillo-Lopez AJ, Janakiraman N, Saven A, Beck TM, Varns C, Shuey S, Czuczman M, Lynch JW, Kolitz JE, Jain V: Extended Rituximab (anti-CD20monoclonal antibody) therapy for relapsed or refractory low-grade or follicularnon-Hodgkin's lymphoma. Ann Oncol 10: 655–661, 1999
Molthoff CF, Prinssen HM, Kenemans P, van Hof AC, den Hollander W, Verheijen RH: Escalating protein doses of chimeric monoclonal antibody MOv18 immunoglobulin G inovarian carcinoma patients: A phase I study. Cancer 80: 2712–2720, 1997
Kelley MJ, Linnoila RI, Avis IL, Georgiadis MS, Cuttitta F, Mulshine JL, Johnson BE: Antitumor activity of a monoclonal antibody directed against gastrin-releasingpeptide in patients with small cell lung cancer. Chest 112: 256–261, 1997
Soderlind E, Ohlin M, Carlsson R: Complementarity-determining region (CDR)implantation: A theme of recombination. Immunotechnology 4: 279–285, 1999
Corti A, Barbanti E, Tempest PR, Carr FJ, Marcucci F: Idiotope determiningregions of a mouse monoclonal antibody and its humanized versions. Identification offramework residues that affect idiotype expression. J Mol Biol 235: 53–60, 1994
Tempest PR, Bremner P, Lambert M, Taylor G, Furze JM, Carr FJ, Harris WJ:Reshaping a human monoclonal antibody to inhibit human respiratory syncytial virusinfection in vivo. Biotechnology (N Y) 9: 266–271, 1991
Isaacs JD, Watts RA, Hazleman BL, Hale G, Keogan MT, Cobbold SP, Waldmann H:Humanised monoclonal antibody therapy for rheumatoid arthritis. Lancet 340: 748–752, 1992
Gilliland LK, Walsh LA, Frewin MR, Wise MP, Tone M, Hale G, Kioussis D, Waldmann H: Elimination of the immunogenicity of therapeutic antibodies. J Immunol 162:3663–3671, 1999
Hudson PJ, Souriau C: Engineered antibodies. Nat Med 9: 129–134, 2003
Kellermann SA, Green LL: Antibody discovery: The use of transgenic mice togenerate human monoclonal antibodies for therapeutics. Curr Opin Biotechnol 13: 593–597, 2002
Kretzschmar T, von Ruden T: Antibody discovery: Phage display. Curr Opin Biotechnol 13: 598–602, 2002
Carter P, Merchant AM: Engineering antibodies for imaging and therapy. Curr Opin Biotechnol 8: 449–454, 1997
Hudson PJ: Recombinant antibody fragments. Curr Opin Biotechnol 9: 395–402, 1998
Adams GP, Weiner LM: Radioimmunotherapy of solid tumors: From fairytale toreality. Cancer Biother Radiopharm 16: 9–11, 2001
Eccles SA: Monoclonal antibodies targeting cancer: ‘Magic bullets’ or justthe trigger? Breast Cancer Res 3: 86–90, 2001
Johnson PW: The therapeutic use of antibodies for malignancy. Transfus Clin Biol 8: 255–259, 2001
Bindon CI, Hale G, Waldmann H: Importance of antigen specificity forcomplement-mediated lysis by monoclonal antibodies. Eur J Immunol 18: 1507–1514, 1988
Presta LG, Shields RL, Namenuk AK, Hong K, Meng YG: Engineering therapeuticantibodies for improved function. Biochem Soc Trans 30: 487–490, 2002
Presta LG, Shields RL, Namenuk AK, Hong K, Meng YG: Engineering therapeuticantibodies for improved function. Biochem Soc Trans 30: 487–490, 2002
Presta LG: Engineering antibodies for therapy. Curr Pharm Biotechnol 3:237–256, 2002
Ravetch JV, Bolland S: Ig G Fc receptors. Annu Rev Immunol 19: 275–290, 2001
Batra SK, Jain M, Wittel UA, Chauhan SC, Colcher D: Pharmacokinetics andbiodistribution of genetically engineered antibodies. Curr Opin Biotechnol 13: 603–608, 2002
Dayan AD: Safety evaluation of biological and biotechnology-derivedmedicines. Toxicology 105: 59–68, 1995
Johnson PW: The therapeutic use of antibodies for malignancy. Transfus Clin Biol 8: 255–259, 2001
Lipsky MS, Sharp LK: From idea to market: The drug approval process. J Am Board Fam Pract 14: 362–367, 2001
Waldmann TA, Levy R, Coller BS: Emerging Therapies: Spectrum of Applicationsof Monoclonal Antibody Therapy. Hematology (Am Soc Hematol Educ Program)394–408, 2000
Waldmann H, Gilliland LK, Cobbold P, Hale G: Immunotherapy. In: Paul W (ed)Fundamental Immunology, 4th ed. Lippincott, Williams and Wilkins, 2000, Philadelphia, pp.1511–1529
Dyer MJ, Hale G, Hayhoe FG, Waldmann H: Effects of CAMPATH-1 antibodies invivo in patients with lymphoid malignancies: Influence of antibody isotype. Blood 73:1431–1439, 1989
Hale G, Clark M, Waldmann H: Therapeutic potential of rat monoclonalantibodies: Isotype specificity of antibody-dependent cell-mediated cytotoxicity withhuman lymphocytes. J Immunol 134: 3056–3061, 1985
Hale G, Swirsky D, Waldmann H, Chan LC: Reactivity of rat monoclonalantibody CAMPATH-1 with human leukaemia cells and its possible application for autologousbone marrow transplantation. Br J Haematol 60: 41–48, 1985
Green SK, Karlsson MC, Ravetch JV, Kerbel RS: Disruption of cell-celladhesion enhances antibody-dependent cellular cytotoxicity: Implications forantibody-based therapeutics of cancer. Cancer Res 62: 6891–6900, 2002
Kipps TJ, Parham P, Punt J, Herzenberg LA: Importance of immunoglobulinisotype in human antibody-dependent, cell-mediated cytotoxicity directed by murinemonoclonal antibodies. J Exp Med 161: 1–17, 1985
Manches O, Lui G, Chaperot L, Gressin R, Molens JP, Jacob MC, Sotto JJ, Leroux D, Bensa JC, Plumas J: In vitro mechanisms of action of rituximab on primarynon-Hodgkin lymphomas. Blood 101: 949–954, 2003
Naundorf S, Preithner S, Mayer P, Lippold S, Wolf A, Hanakam F, Fichtner I, Kufer P, Raum T, Riethmuller G, Baeuerle PA, Dreier T: In vitro and in vivo activity of MT201, a fully human monoclonal antibody for pancarcinoma treatment. Int J Cancer 100:101–110, 2002
Popkov M, Sidrac-Ghali S, Lusignan Y, Lemieux S, Mandeville R: Inhibition oftumour growth and metastasis of human fibrosarcoma cells HT-1080 by monoclonal antibody BCD-F9. Eur J Cancer 37: 2484–2492, 2001
Ratzinger G, Reagan JL, Heller G, Busam KJ, Young JW: Differential CD52expression by distinct myeloid dendritic cell subsets: Implications for alemtuzumabactivity at the level of antigen presentation in allogeneic graft-host interactions intransplantation. Blood 101: 1422–1429, 2003
Kim KM, Shin EY, Moon JH, Heo TH, Lee JY, Chung Y, Lee YJ, Cho HM, Shin SU, Kang CY: Both the epitope specificity and isotype are important in the antitumor effect ofmonoclonal antibodies against Her-2/neu antigen. Int J Cancer 102: 428–434, 2002
Clynes RA, Towers TL, Presta LG, Ravetch JV: Inhibitory Fc receptorsmodulate in vivo cytoxicity against tumor targets. Nat Med 6: 443–446, 2000
Clynes RA, Towers TL, Presta LG, Ravetch JV: Inhibitory Fc receptorsmodulate in vivo cytoxicity against tumor targets. Nat Med 6: 443–446, 2000
Maloney DG: Preclinical and phase I and II trials of rituximab. Semin Oncol26: 74–78, 1999
Maloney DG: Mechanism of action of rituximab. Anticancer Drugs 12(Suppl 2):S1–S4, 2001
Ratzinger G, Reagan JL, Heller G, Busam KJ, Young JW: Differential CD52expression by distinct myeloid dendritic cell subsets: Implications for alemtuzumabactivity at the level of antigen presentation in allogeneic graft-host interactions intransplantation. Blood 101: 1422–1429, 2003
Janeway CA, Traverse P, Walport M, Capra JD: Immunobiology: The immunesyustem in health and disease, 4th ed. Elsevier Science, London, 1999, pp 307–308
Ross J, Gray K, Schenkein D, Greene B, Gray GS, Shulok J, Worland PJ, Celniker A, Rolfe M: Antibody-based therapeutics in oncology. Expert Rev Anticancer Ther3: 107–121, 2003
Glennie MJ, Johnson PW: Clinical trials of antibody therapy. Immunol Today21: 403–410, 2000
Green MC, Murray JL, Hortobagyi GN: Monoclonal antibody therapy for solidtumors. Cancer Treat Rev 26: 269–286, 2000
Prodinger WM, Wurzner R, Erdei A, Dierich MP: Complement. In Paul W (ed)Fundamental Immunology, 4th ed. Lippincott, Williams and Wilkins, Philadelphia, 1999, pp967–995
Dyer MJ, Hale G, Hayhoe FG, Waldmann H: Effects of CAMPATH-1 antibodies invivo in patients with lymphoid malignancies: Influence of antibody isotype. Blood 73:1431–1439, 1989
Maloney DG: Mechanism of action of rituximab. Anticancer Drugs 12(Suppl 2):S1–S4, 2001
Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA:Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin). Semin Oncol 26: 60–70, 1999
Spiridon CI, Ghetie MA, Uhr J, Marches R, Li JL, Shen GL, Vitetta ES:Targeting multiple Her-2 epitopes with monoclonal antibodies results in improvedantigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res 8: 1720–1730, 2002
Anderson DR, Grillo-Lopez A, Varns C, Chambers KS, Hanna N: Targetedanti-cancer therapy using rituximab, a chimaeric anti-CD20 antibody (IDEC-C2B8) in thetreatment of non-Hodgkin's B-cell lymphoma. Biochem Soc Trans 25: 705–708, 1997
Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri GM, Bernasconi S, Tedesco F, Rambaldi A, Introna M: Biologic response of B lymphoma cells to anti-CD20monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated celllysis. Blood 95: 3900–3908, 2000
Harjunpaa A, Junnikkala S, Meri S: Rituximab (anti-CD20) therapy of B-celllymphomas: Direct complement killing is superior to cellular effector mechanisms. Scand JImmunol 51: 634–641, 2000
Clynes RA, Towers TL, Presta LG, Ravetch JV: Inhibitory Fc receptorsmodulate in vivo cytoxicity against tumor targets. Nat Med 6: 443–446, 2000
Anderson DR, Grillo-Lopez A, Varns C, Chambers KS, Hanna N: Targetedanti-cancer therapy using rituximab, a chimaeric anti-CD20 antibody (IDEC-C2B8) in thetreatment of non-Hodgkin's B-cell lymphoma. Biochem Soc Trans 25: 705–708, 1997
Maloney DG: Mechanism of action of rituximab. Anticancer Drugs 12(Suppl 2):S1–S4, 2001
Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A: Cytokine-releasesyndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte countsafter treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 94:2217–2224, 1999
Crowe JS, Hall VS, Smith MA, Cooper HJ, Tite JP: Humanized monoclonalantibody CAMPATH-1H: Myeloma cell expression of genomic constructs, nucleotide sequence ofcDNA constructs and comparison of effector mechanisms of myeloma and Chinese hamster ovarycell-derived material. Clin Exp Immunol 87: 105–110, 1992
Dyer MJ, Hale G, Hayhoe FG, Waldmann H: Effects of CAMPATH-1 antibodies invivo in patients with lymphoid malignancies: Influence of antibody isotype. Blood 73:1431–1439, 1989
Dyer MJ: The role of CAMPATH-1 antibodies in the treatment of lymphoidmalignancies. Semin Oncol 26: 52–57, 1999
Hale G, Swirsky DM, Hayhoe FG, Waldmann H: Effects of monoclonalanti-lymphocyte antibodies in vivo in monkeys and humans. Mol Biol Med 1: 321–334, 1983
Hale G, Clark M, Waldmann H: Therapeutic potential of rat monoclonalantibodies: Isotype specificity of antibody-dependent cell-mediated cytotoxicity withhuman lymphocytes. J Immunol 134: 3056–3061, 1985
Woynarowska BA, Woynarowski JM: Preferential targeting of apoptosis in tumorversus normal cells. Biochim Biophys Acta 1587: 309–317, 2002
Makin G, Dive C: Apoptosis and cancer chemotherapy. Trends Cell Biol 11:S22–S26, 2001
Pettit SJ, Seymour K, O'Flaherty E, Kirby JA: Immune selection in neoplasia:Towards a microevolutionary model of cancer development. Br J Cancer 82: 1900–1906, 2000
White CA, Weaver RL, Grillo-Lopez AJ: Antibody-targeted immunotherapy fortreatment of malignancy. Annu Rev Med 52: 125–145, 2001
Ross JS, Gray K, Gray GS, Worland PJ, Rolfe M: Anticancer antibodies. Am JClin Pathol 119: 472–485, 2003
Adams GP, Weiner LM: Radioimmunotherapy of solid tumors: From fairytale toreality. Cancer Biother Radiopharm 16: 9–11, 2001
Boerman OC, van Schaijk FG, Oyen WJ, Corstens FH: Pretargetedradioimmunotherapy of cancer: Progress step by step. J Nucl Med 44: 400–411, 2003
Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, Roy S, Sridhara R, Rahman A, Williams G, Pazdur R: Approval summary: Gemtuzumab ozogamicin in relapsed acutemyeloid leukemia. Clin Cancer Res 7: 1490–1496, 2001
Pangalis GA, Dimopoulou MN, Angelopoulou MK, Tsekouras C, Vassilakopoulos TP, Vaiopoulos G, Siakantaris MP: Campath-1H (anti-CD52) monoclonal antibody therapy inlymphoproliferative disorders. Med Oncol 18: 99–107, 2001
Sievers EL: Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukaemia in first relapse. Expert Opin Biol Ther 1: 893–901, 2001
Grana C, Chinol M, Robertson C, Mazzetta C, Bartolomei M, De Cicco C, Fiorenza M, Gatti M, Caliceti P, Paganelli G: Pretargeted adjuvant radioimmunotherapy withyttrium-90-biotin in malignant glioma patients: A pilot study. Br J Cancer 86: 207–212, 2002
Knox SJ, Goris ML, Tempero M, Weiden PL, Gentner L, Breitz H, Adams GP, Axworthy D, Gaffigan S, Bryan K, Fisher DR, Colcher D, Horak ID, Weiner LM: Phase II trialof yttrium-90-DOTA-biotin pretargeted by NR-LU-10 antibody/streptavidin in patients withmetastatic colon cancer. Clin Cancer Res 6: 406–414, 2000
Yao Z, Zhang M, Axworthy DB, Wong KJ, Garmestani K, Park L, Park CW, Mallett RW, Theodore LJ, Yau EK, Waldmann TA, Brechbiel MW, Paik CH, Pastan I, Carrasquillo JA:Radioimmunotherapy of A431 xenografted mice with pretargeted B3 antibody-streptavidin and(90)Y-labeled 1, 4, 7, 10-tetraazacyclododecane-N, N′, N″, N″-tetraacetic acid(DOTA)-biotin. Cancer Res 62: 5755–5760, 2002
Press OW, Corcoran M, Subbiah K, Hamlin DK, Wilbur DS, Johnson T, Theodore L, Yau E, Mallett R, Meyer DL, Axworthy D: A comparative evaluation of conventional andpretargeted radioimmunotherapy of CD20-expressing lymphoma xenografts. Blood 98:2535–2543, 2001
Goshorn S, Sanderson J, Axworthy D, Lin Y, Hylarides M, Schultz J:Preclinical evaluation of a humanized NR-LU-10 antibody-streptavidin fusion protein forpretargeted cancer therapy. Cancer Biother Radiopharm 16: 109–123, 2001
Axworthy DB, Reno JM, Hylarides MD, Mallett RW, Theodore LJ, Gustavson LM, Su F, Hobson LJ, Beaumier PL, Fritzberg AR: Cure of human carcinoma xenografts by a singledose of pretargeted yttrium-90 with negligible toxicity. Proc Natl Acad Sci USA 97:1802–1807, 2000
Paganelli G, Bartolomei M, Ferrari M, Cremonesi M, Broggi G, Maira G, Sturiale C, Grana C, Prisco G, Gatti M, Caliceti P, Chinol M: Pre-targeted locoregionalradioimmunotherapy with 90Y-biotin in glioma patients: Phase I study and preliminarytherapeutic results. Cancer Biother Radiopharm 16: 227–235, 2001
Breitz HB, Weiden PL, Beaumier PL, Axworthy DB, Seiler C, Su FM, Graves S, Bryan K, Reno JM: Clinical optimization of pretargeted radioimmunotherapy withantibody-streptavidin conjugate and 90Y-DOTA-biotin. J Nucl Med 41: 131–140, 2000
Knox SJ, Goris ML, Tempero M, Weiden PL, Gentner L, Breitz H, Adams GP, Axworthy D, Gaffigan S, Bryan K, Fisher DR, Colcher D, Horak ID, Weiner LM: Phase II trialof yttrium-90-DOTA-biotin pretargeted by NR-LU-10 antibody/streptavidin in patients withmetastatic colon cancer. Clin Cancer Res 6: 406–414, 2000
Paganelli G, Bartolomei M, Ferrari M, Cremonesi M, Broggi G, Maira G, Sturiale C, Grana C, Prisco G, Gatti M, Caliceti P, Chinol M: Pre-targeted locoregionalradioimmunotherapy with 90Y-biotin in glioma patients: Phase I study and preliminarytherapeutic results. Cancer Biother Radiopharm 16: 227–235, 2001
Weiden PL, Breitz HB: Pretargeted radioimmunotherapy (PRIT) for treatment ofnon-Hodgkin's lymphoma (NHL). Crit Rev Oncol Hematol 40: 37–51, 2001
Gura T: Therapeutic antibodies: Magic bullets hit the target. Nature 417:584–586, 2002
Gura T: Therapeutic antibodies: Magic bullets hit the target. Nature 417:584–586, 2002
Chadd HE, Chamow SM: Therapeutic antibody expression technology. Curr Opin Biotechnol 12: 188–194, 2001
Reavy B, Ziegler A, Diplexcito J, Macintosh SM, Torrance L, Mayo M:Expression of functional recombinant antibody molecules in insect cell expression systems.Protein Expr Purif 18: 221–228, 2000
Sanchez L, Ayala M, Freyre F, Pedroso I, Bell H, Falcon V, Gavilondo JV:High cytoplasmic expression in E. coli, purification, and in vitro refolding of a singlechain Fv antibody fragment against the hepatitis B surface antigen. J Biotechnol 72:13–20, 1999
Allison DE, Gourlay SG, Koren E, Miller RM, Fox JA: Pharmacokinetics ofrhu MAb CD18, a recombinant humanised monoclonal antibody fragment to CD18, in normalhealthy human volunteers. Bio Drugs 16: 63–70, 2002
Looareesuwan S, Sjostrom L, Krudsood S, Wilairatana P, Porter RS, Hills F, Warrell DA: Polyclonal anti-tumor necrosis factor-alpha Fab used as an ancillary treatmentfor severe malaria. Am J Trop Med Hyg 61: 26–33, 1999
Juweid M, Sharkey RM, Behr TM, Swayne LC, Dunn R, Ying Z, Siegel JA, Hansen HJ, Goldenberg DM: Clinical evaluation of tumor targeting with the anticarcinoembryonicantigen murine monoclonal antibody fragment, MN-14 F(ab)2. Cancer 78: 157-168, 1996
Sharkey RM, Goldenberg DM, Vagg R, Pawlyk D, Wong GY, Siegel JA, Murthy S, Levine GM, Izon D, Gascon P: Phase I clinical evaluation of a new murine monoclonalantibody (Mu-9) against colon-specific antigen-p for targeting gastrointestinalcarcinomas. Cancer 73: 864–877, 1994
Wada K, Watanabe T, Tadokoro M, Sakamoto J, Murayama H, Sakuma S, Takagi H:[Radioimmunodetection of colorectal cancer, using anti-CEA monoclonal antibody CEA 102:Whole Ig G versus F(ab')2 fragments]. Nippon Geka Gakkai Zasshi 93: 266–273, 1992
Colcher D, Bird R, Roselli M, Hardman KD, Johnson S, Pope S, Dodd SW, Pantoliano MW, Milenic DE, Schlom J: In vivo tumor targeting of a recombinant single-chainantigen-binding protein. J Natl Cancer Inst 82: 1191–1197, 1990
Hudson PJ: Recombinant antibody fragments. Curr Opin Biotechnol 9:395–402, 1998
Swartz JR: Advances in Escherichia coli production of therapeutic proteins.Curr Opin Biotechnol 12: 195–201, 2001
De Bernardez CE: Protein refolding for industrial processes. Curr Opin Biotechnol 12: 202–207, 2001
Swartz JR: Advances in Escherichia coli production of therapeutic proteins.Curr Opin Biotechnol 12: 195–201, 2001
Andersen DC, Krummen L: Recombinant protein expression for therapeuticapplications. Curr Opin Biotechnol 13: 117–123, 2002
Houdebine LM: Antibody manufacture in transgenic animals and comparisonswith other systems. Curr Opin Biotechnol 13: 625–629, 2002
Chu L, Robinson DK: Industrial choices for protein production bylarge-scale cell culture. Curr Opin Biotechnol 12: 180–187, 2001
Chu L, Robinson DK: Industrial choices for protein production bylarge-scale cell culture. Curr Opin Biotechnol 12: 180–187, 2001
Hudson PJ: Recombinant antibody fragments. Curr Opin Biotechnol 9:395–402, 1998
BD Kelley, Biochemical engineering: Bioprocessing of therapeutic proteins.Curr Opin Biotechnol 12: 173–174, 2001
Laken HA, Leonard MW: Understanding and modulating apoptosis in industrialcell culture. Curr Opin Biotechnol 12: 175–179, 2001
Grunfeld H, Moore P: Reduction of bovine immunoglobulin contamination frommonoclonal antibodies by SOURCE 15PHE chromatography. J Immunol Methods 201: 233–241, 1997
Jiskoot W, Van Hertrooij JJ, Hoven AM, Klein Gebbinck JW, Van der Velden-de Groot, Crommelin DJ, Beuvery EC: Preparation of clinical grade monoclonal antibodies fromserum-containing cell culture supernatants. J Immunol Methods 138: 273–283, 1991
Laken HA, Leonard MW: Understanding and modulating apoptosis in industrialcell culture. Curr Opin Biotechnol 12: 175–179, 2001
Siegel R, Naveh D: Large scale downstream processing of monoclonalantibodies. Bioseparation 1: 351–359, 1991
Isaacs JD, Watts RA, Hazleman BL, Hale G, Keogan MT, Cobbold SP, Waldmann H: Humanised monoclonal antibody therapy for rheumatoid arthritis. Lancet 340: 748–752, 1992
Gilliland LK, Walsh LA, Frewin MR, Wise MP, Tone M, Hale G, Kioussis D, Waldmann H: Elimination of the immunogenicity of therapeutic antibodies. J Immunol 162:3663–3671, 1999
Batra SK, Jain M, Wittel UA, Chauhan SC, Colcher D: Pharmacokinetics andbiodistribution of genetically engineered antibodies. Curr Opin Biotechnol 13: 603–608, 2002
Dayan AD: Safety evaluation of biological and biotechnology-derivedmedicines. Toxicology 105: 59–68, 1995
Lipsky MS, Sharp LK: From idea to market: the drug approval process. J Am Board Fam Pract 14: 362–367, 2001
Ross J, Gray K, Schenkein D, Greene B, Gray GS, Shulok J, Worland PJ, Celniker A, Rolfe M: Antibody-based therapeutics in oncology. Expert Rev Anticancer Ther3: 107–121, 2003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanner, J.E. Designing antibodies for oncology. Cancer Metastasis Rev 24, 585–598 (2005). https://doi.org/10.1007/s10555-005-6197-x
Issue Date:
DOI: https://doi.org/10.1007/s10555-005-6197-x