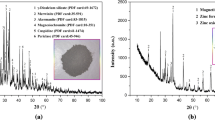
Abstract
Stainless steel dust as the main solid waste produced in the process of stainless steel smelting has the characteristics of high basicity, low treatment efficiency, and low recovery. In this study, chromium-containing slag with low basicity was added to stainless steel dust on the basis of carbothermal reduction. Through the study of the effect of single factor of reduction process and multiple factors based on response surface method (RSM) on the recovery of metal Fe, Cr, and Ni, the efficient reduction of valuable metal components in stainless steel dust was realized. The results show that the addition of chromium-containing slag and reduction temperature have a significant impact on the metal recovery of the reduction product, and there is a significant interaction between the metal recovery of Fe, Cr, and Ni in the reduction product. The optimum process parameters are as follows: the addition of chromium-containing slag is 6%, the reduction temperature is 1425 °C, the reduction time is 28 min, and the molar ratio of fixed carbon to metal oxide (FC/O) is 0.9. The predicted recoveries of Fe, Cr, and Ni were 94%, 93%, and 97%, respectively.
Graphical abstract









Similar content being viewed by others
References
Jung SS, Kim GB, Sohn I (2017) Understanding the solidification of stainless steel slag and dust mixtures. J Am Ceram Soc 100:3771–3783. https://doi.org/10.1111/jace.14891
Habib A, Bhatti HN, Iqbal M (2019) Metallurgical processing strategies for metals recovery from industrial slags. Z Phys Chem 234:201–231. https://doi.org/10.1515/zpch-2019-0001
Li YS, Xue ZL, Tang E, Liu Q, Wang WX, Zhao DN (2012) Research on the recovery of secondary iron-bearing dust in synergistic reduction of carbon composite pellets. Adv Mater Res 524–527:2031–2036. https://doi.org/10.4028/www.scientific.net/AMR.524-527.2031
Zhang HW, Hong X (2011) An overview for the utilization of wastes from stainless steel industries. Resour Conserv Recycl 55:745–754. https://doi.org/10.1016/j.resconrec.2011.03.005
Rieger J, Schenk J (2019) Residual processing in the European steel industry: a technological overview. J Sustain Metall 5:295–309. https://doi.org/10.1007/s40831-019-00220-2
Chai LY, Huang SH, Yang ZH, Peng B, Huang Y, Chen YH (2009) Cr (VI) remediation by indigenous bacteria in soils contaminated by chromium-containing slag. J Hazard Mater 167:516–522. https://doi.org/10.1016/j.jhazmat.2009.01.030
Lin Y, Yan BJ, Fabritius T, Shu QF (2020) Immobilization of chromium in stainless steel slag using low zinc electric arc furnace dusts. Metall Mater Trans B 51:763–775. https://doi.org/10.1007/s11663-020-01777-0
Peng J, Peng B, Yu D, Tang MT, Souza N, Kozinski JA, Lobel J (2003) Thermo-analytical study on stainless steelmaking dust. J Cent South Univ T 4:301–306. https://doi.org/10.1007/s11771-003-0028-4
Sokchol R, Chu MS, Chen SY, Liu ZG, Hong H (2016) Self-reduction mechanism of coal composite stainless steel dust hot briquette. J Iron Steel Res Int 23:314–321. https://doi.org/10.1016/S1006-706X(16)30051-6
Liu PJ, Liu ZG, Chu MS, Tang J, Gao LH, Yan RJ (2021) Green and efficient utilization of stainless steel dust by synergistic reduction and self-pulverization. J Hazard Mater 413:125403. https://doi.org/10.1016/j.jhazmat.2021.125403
Calvo G, Valero A, Valero A (2017) Assessing maximum production peak and resource availability of non-fuel mineral resources: Analyzing the influence of extractable global resources. Resour Conserv Recycl 125:208–217. https://doi.org/10.1016/j.resconrec.2017.06.009
Holappa L, Kekkonen M, Jokilaakso A, Koskinen J (2021) A review of circular economy prospects for stainless steelmaking slags. J Sustain Metall 2021:1–12. https://doi.org/10.1007/s40831-021-00392-w
De Araújo JA, Schalch V (2014) Recycling of electric arc furnace (EAF) dust for use in steel making process. J Mater Res Technol 3:274–279. https://doi.org/10.1016/j.jmrt.2014.06.003
Kim G, Sohn I (2018) Selective metal cation concentration during the solidification of stainless steel EAF dust and slag mixtures from high temperatures for increased Cr recovery. J Hazard Mater 359:174–185. https://doi.org/10.1016/j.jhazmat.2018.07.053
Lindvall M, So L, Mahdi M, Bolen J, Nell J, Metcalfe D, Mostaghel S, Sundqvist O (2019) Stabilization of stainless steel slag via air granulation. J Sustain Metall 5:157–171. https://doi.org/10.1007/s40831-019-00212-2
Ahmed HM, Viswanathan N, Bjorkman B (2014) Composite pellets-a potential raw material for iron-making. Steel Res Int 85:293–306. https://doi.org/10.1002/srin.201300072
Zhang YY, Shi XF, Qi YH, Zou ZS, Guo YH (2015) Slag composition mechanism based reduction and smelting of carbon composite bauxite pellets. Kang T’ieh/Iron and Steel (Peking) 50:17–21. https://doi.org/10.13228/j.boyuan.issn0449-749x.20140073.
Wu XR, Dong XM, Wang RT, Lv HH (2016) Crystallization behaviour of chromium in stainless steel slag: effect of FeO and basicity. J Residuals Sci Technol 13:S57-S62. https://doi.org/10.12783/issn.1544-8053/13/2/S10.
Tang E, Liang XZ, Li JY, Zhou Q (2013) Research on the process of iron-bearing dust’s self-reduction to produce iron nuggets. Adv Mater 746:505–510. https://doi.org/10.4028/www.scientific.net/AMR.746.505
Doronin IE, Svyazhin AG (2011) Commercial methods of recycling dust from steelmaking. Metallurgist 54:673–681. https://doi.org/10.1007/s11015-011-9356-z
Laforest G, Duchesne J (2006) Characterization and leachability of electric arc furnace dust made from remelting of stainless steel. J Hazard Mater 135:156–164. https://doi.org/10.1016/j.jhazmat.2005.11.037
Ye GZ, Burström E, Kuhn M, Piret J (2003) Reduction of steel-making slags for recovery of valuable metals and oxide materials. Scand J Metall 32:7–14. https://doi.org/10.1034/j.1600-0692.2003.00526.x
Ji YL, Shen SB, Liu JH, Guo JL, Zhao YS (2016) Mechanisms involved in the roasting of pellets composed of stainless steel slag and sodium hydroxide to extract chromium. ISIJ Int 56:1751–1757. https://doi.org/10.2355/isijinternational.ISIJINT-2016-320
Long HM, Meng QM, Wang P, Chun TJ, Yao YL (2015) Preparation of chromium-iron metal powder from chromium slag by reduction roasting and magnetic separation. J Iron Steel Res Int 22:771–776. https://doi.org/10.1016/S1006-706X(15)30070-4
Inoue R, Sato Y, Takasaki Y, Shibayama A (2016) Immobilization of hexavalent chromium in stainless steelmaking slag: proceedings of the 10th international conference on molten slags, fluxes and salts. Springer International Publishing. ISBN: 9781119308768.
Gu FQ, Zhang YB, Su ZJ, Tu YK, Liu S, Jiang T (2021) Recovery of chromium from chromium-bearing slags produced in the stainless-steel smelting: A review. J Clean Prod 296:126467. https://doi.org/10.1016/j.jclepro.2021.126467
Omran M, Fabritius T (2019) Utilization of blast furnace sludge for the removal of zinc from steelmaking dusts using microwave heating. Sep Purif Technol 210:867–884. https://doi.org/10.1016/j.seppur.2018.09.010
Bahrami M, Amiri MJ, Bagheri F (2019) Optimization of the lead removal from aqueous solution using two starch based adsorbents: Design of experiments using response surface methodology (RSM). J Environ Chem Eng 7:102793. https://doi.org/10.1016/j.jece.2018.11.038
Abdulgader M, Yu QJ, Zinatizadeh AA, Williams P, Rahimi Z (2020) Application of response surface methodology (RSM) for process analysis and optimization of milk processing wastewater treatment using multistage flexible fiber biofilm reactor. J Environ Chem Eng 8:103797. https://doi.org/10.1016/j.jece.2020.103797
Zhang L, Guo XY, Tian QH, Qin H (2021) Selective separation of arsenic from high-arsenic dust in the NaOH-S system based on response surface methodology. J Sustain Metall 7:684–703. https://doi.org/10.1007/s40831-021-00372-0
Guo C, Ding L, Jin X, Zhang H, Zhang D (2021) Application of response surface methodology to optimize chromium (VI) removal from aqueous solution by cassava sludge-based activated carbon. J Environ Chem Eng 9:104785. https://doi.org/10.1016/j.jece.2020.104785
Mwanat HM, Kasongo KB (2021) Cobalt dissolution from concentrate in sulfuric acid—ferrous sulfate system: process parameters optimization by response surface methodology (RSM). J Sustain Metall 7:1838–1851. https://doi.org/10.1007/s40831-021-00460-1
Chang T, Shen ZX, Ma CL, Lu JQ, Huang Y, Savita KPV, Nathalie DG, Rino M (2021) Process optimization of plasma-catalytic formaldehyde removal using MnOx-Fe2O3 catalysts by response surface methodology. J Environ Chem Eng 9:105773. https://doi.org/10.1016/j.jece.2021.105773
Bashir MJK, Aziz HA, Yusoff MS, Adlan MN (2010) Application of response surface methodology (RSM) for optimization of ammoniacal nitrogen removal from semi-aerobic landfill leachate using ion exchange resin. Desalination 254:154–161. https://doi.org/10.1016/j.desal.2009.12.002
Rath SS, Sahoo H, Das B (2013) Optimization of flotation variables for the recovery of hematite particles from BHQ ore. Int J Miner Metall Mater 20:605–611. https://doi.org/10.1007/s12613-013-0773-9
Azizi D, Shafaei SZ, Noaparast M, Abdollahi H (2012) Modeling and optimization of low-grade Mn bearing ore leaching using response surface methodology and central composite rotatable design. Trans Nonferrous Met Soc China 22:2295–2305. https://doi.org/10.1016/S1003-6326(11)61463-5
Liu BG, Peng JH, Wan RD, Zhang LB, Guo SH, Zhang SM (2011) Optimization of preparing V2O5 by calcination from ammonium metavanadate using response surface methodology. Trans Nonferrous Met Soc China 21:673–678. https://doi.org/10.1016/S1003-6326(11)60764-4
Sokchol R, Chu MS (2015) Separation of metal nugget from self-reduced product of coal composite stainless steel dust briquette. ISIJ Int 55:1565–1572. https://doi.org/10.2355/isijinternational.ISIJINT-2014-845
Iron ores-Determination of total iron content-Titanium (III) chloride reduction potassium dichromate titration methods (routine methods) (GB/T 6730.65–2009)
Iron ores-Determination of chromium content-Flame atomic absorption spectrometric method (GB/T 6730.57–2004)
Iron ores-Determination of nickel content-Flame atomic absorption spectrometric method (GB/T 6730.60–2005)
Methods for chemical analysis of iron ores: The potassium dichromate volumetric method for the determination of iron (II) content (GB/T 6730.8–1986)
Acknowledgements
The authors are especially grateful to the National Natural Science Foundation of China (No. 51974077) and Xingliao Talent Plan (No. XLYC1902118), and special thanks are due to the instrumental analysis from Analytical and Testing Center, Northeastern University.
Author information
Authors and Affiliations
Contributions
PL: Investigation, Writing—original draft. ZL and MC: Writing—review and editing. RY: Data curation. FL: Photo editing. JT: Methodology, Validation. JF: Investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was M Akbar Rhamdhani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, P., Liu, Z., Chu, M. et al. Efficient Utilization of Stainless Steel Dust and Chromium-Containing Slag by Carbothermal Direct Reduction: Synergistic Mechanism and Optimization Analysis. J. Sustain. Metall. 8, 1877–1891 (2022). https://doi.org/10.1007/s40831-022-00610-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00610-z