Abstract
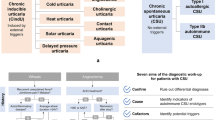
Urticaria can manifest at any age, including infants and young children. Urticaria is one of the most prevalent skin diseases in childhood. As in adults, a distinction is made between acute and chronic urticaria, with chronic urticaria further classified into chronic spontaneous urticaria and inducible urticaria. According to the current German S3 guideline for classification, diagnosis, and treatment of urticaria, existing literature suggests that the prevalence, disease characteristics, causes, and also the response to treatment are very similar in children and adults. The clinical hallmark of urticaria is the subjective sensation of itch. In young children who may have difficulty expressing itching, it is crucial to observe their scratching behavior during clinical examinations. Particularly in children, mastocytosis and autoinflammatory syndromes (cryopyrin-associated periodic syndromes [CAPS], especially Muckle–Wells syndrome and childhood Still’s disease) are important differential diagnoses. Autoinflammatory syndromes are characterized by additional symptoms such as fever, bone pain, muscle pain, and joint complaints. Cryopyrin-associated periodic syndromes usually manifest in infancy, so that these diseases must be considered, especially if cold-associated urticarial skin lesions are present. Appropriate and early treatment can prevent serious sequelae. In maculopapular mastocytosis (urticaria pigmentosa), reddish macules are characteristic for the disease; especially after elicitation of the Darier’s sign, differentiation from urticaria can be difficult, but the macules are permanent. Clinically, wheals and/or angioedema are found similarly to adults, indicating that mast cell-induced angioedema is also associated with childhood urticaria. In the case of exclusive angioedema, hereditary angioedema must also be considered, which usually manifests for the first time during puberty, often linked to hormone preparation usage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most common skin diseases in childhood is urticaria, which can also occur in infancy and early childhood [1]. As in adults, a distinction is made between acute urticaria and chronic urticaria (CU), with CU being subdivided into chronic spontaneous urticaria (CSU) and inducible urticaria (CindU) [2, 3]. According to the German S3 guideline on the classification, diagnosis, and treatment of urticaria [2, 3], it can be assumed that the prevalence, disease characteristics, causes, and also the response to treatment are very similar in children and adults.
Epidemiology of urticaria in children
Overall, urticaria is one of the most prevalent skin diseases in childhood, with acute urticaria tending to be more common in children than in adults, whereas chronic urticaria is less common [1].
For this purpose, data from two German birth cohort studies (LISAplus 3097 newborns from 1997–1999 and GINAplus 5991 newborns from September 1995–June 1998) were published. The diagnosis of urticaria was taken from questionnaires filled out by the families. In the first 10 years of life, urticaria had occurred in 14.5% of boys and 16.2% of girls (cumulative prevalence), and the annual incidence was reported to be 1% [4].
A 2017 Korean study on 4076 children aged 4–13 years, who participated in a prospective atopy study, estimated the childhood prevalence of chronic urticaria to be approximately 1.8% [5]. Risk factors for acute urticaria included atopic diseases in the family, specifically allergic asthma, allergic rhinoconjunctivitis, and atopic dermatitis in the parents. However, this was not found to be a risk factor for CSU [5].
Clinical presentation of childhood urticaria
Clinically, urticaria in children, as in adults, is characterized by pruritic, transient wheals, persisting for less than 24 h locoregionally. Concurrent or isolated angioedema in the form of subcutaneous swelling, especially on the extremities or face, may also be present. Because infants and young children cannot articulate their itching, physical examination and observation during the assessment (scratching behavior) is of high importance. Scratch excoriations are not typical for urticaria, as wheals are more likely to be rubbed. The parents’ information regarding the location of wheals, duration of the individual wheal and itching is essential for taking the medical history, while photographs of skin lesions can be extremely helpful.
If wheals persist for more than 24 h, alternative conditions such as urticarial vasculitis [6] or neutrophilic urticarial dermatosis (NUD) [7] should be excluded by histologic examination of a skin biopsy. Usually, typical wheals do not leave residuals. Residuals in the sense of livid and brownish macules after disappearance of a wheal can also be an indication of diseases such as urticarial vasculitis/NUD/autoinflammation [7].
Angioedema presents as deeper swelling that typically resolves within approximately 24–72 h. If localized in the mouth and larynx area, life-threatening airway obstructions may occur, requiring immediate medical intervention.
School performance of children with CSU is decreased compared with children without urticaria, and CSU also significantly decrease children’s quality of life [6, 8], pointing out the importance of effectively managing urticaria in children with appropriate treatment.
Diagnostic workup in urticaria in childhood
Diagnostic workup in acute urticaria
In the case of acute urticaria, the current German S3 guidelines do not generally recommend routine diagnostics in children, similar to adults, due to the self-limiting nature of the disease [2, 3]. Only when there are anamnestic or clinical indications of an underlying cause such as infection or triggering by drugs or food, allergy tests and specific laboratory analyses including microbiological diagnostics should be considered in individual cases. According to the literature, viral infections, especially of the upper respiratory tract (e.g., due to adenoviruses, enteroviruses, rotaviruses, RSV, Epstein–Barr virus, cytomegalovirus), can be a trigger of acute urticaria; bacterial infections (especially with streptococci, mycoplasma pneumoniae, and Helicobacter pylori) may also play a role [9]. Because of possible, but with adequate therapy avoidable sequelae, especially after infections with Streptococcus pyogenes group A (scarlet fever), such infections should not be overlooked. Parasitic infections with Enterobius vermicularis, Anisakis simplex and other worms, but also Plasmodium falciparum and Blastocystis hominis, may also be associated with acute urticaria [9, 10], with frequency varying by patient origin. Eosinophilia may indicate parasitic disease, and diagnosis is made by examination of perianal scrapings (strips of adhesive film) for oxyuriasis or stool analysis for parasites.
In a retrospective Taiwanese study, acute urticaria was associated with infection, especially upper respiratory tract infection, in 48.4% of patients who visited an emergency department for urticaria; acute gastroenteritis was also common. In 23.5%, acute urticaria was caused by food and in 11.5% by drugs, especially ibuprofen [11].
Especially in childhood, urticaria can also be triggered by vaccination. According to a retrospective cohort study from China with 220,422 children, anaphylaxis occurred 0–7 days after vaccination with oral poliovirus vaccine or IPV, DTaP, Hib, or PRP in 0.0–0.9 per 100,000 doses and urticaria or angioedema was induced in 0.6–4.6 per 100,000 doses [12]. One review cited an incidence as high as 8.42 anaphylaxis per 1,000,000 vaccinations [13].
Urticaria is one of the most common side effects of coronavirus disease 2019 (COVID-19) vaccination [14, 15], even acute urticaria is more common in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [16].
Wheals can also be a symptom of food allergy and occur in allergic reactions to Hymenoptera venoms [2, 3, 17]. In these situations, however, the reactions are classified as anaphylaxis rather than urticaria. Anaphylaxis is defined by the fact that a trigger can be identified and wheals occur in the context of other symptoms of anaphylaxis.
Diagnostic workup in CSU and CIndU
Hives are triggered by mediators released by mast cells. It is not fully understood which pathophysiological mechanisms lead to mast cell degranulation. Recent evidence suggests that autoimmunity type I (CSUaiTI—or autoallergic CSU with IgE autoantibodies) or autoimmunity type IIb (CSUaiTIIb—due to IgG, IgM, or IgA autoantibodies against IgE/FcεRI on mast cells) are significant [18]. In CSU without a known cause (CSUuc), the mechanisms leading to mast cell degranulation are not known. IgE antibodies responsible for CSUaiTI, which may be directed against TPO or IL-24, for example, are currently not detectable by routine diagnostics [18].
Reportedly, around 50% of children with CSU have a positive autologous serum skin test (ASST) [6], which may be a reason to exclude autoimmune diseases. In a retrospective study from Turkey, thyroid autoantibodies were detected in the serum of 10.7% of 42 children with mast cell-related angioedema [19], and in other studies, the prevalence of thyroid antibodies in children was 6.4% [20]. To rule out collagenoses testing for antinuclear antibodies (ANA) can be performed [21]. Celiac disease should also be excluded; in a study of 79 children with CSU, gluten-sensitive enteropathy was found in 4 (5%) compared with 17/2545 control patients (0.67%). A gluten-free diet also resulted in a complete resolution of urticaria [22]. This association is supported by a population-based cohort study of 28,900 patients with a bioptically proven diagnosis of celiac disease compared with 143,397 reference patients. There was a twofold increased risk of urticaria in celiac disease compared to the control group [23].
According to current understanding, infections, inflammatory processes, food/medication intolerance and stress modify the disease activity of urticaria, but they are usually not the primary cause of the disease. Corresponding examinations are consequently only recommended if there is evidence of such causes [2, 3].
According to a European Academy of Allergy and Clinical Immunology (EAACI) survey, in which 158 pediatricians and pediatric allergists participated, allergy tests and examinations for parasites are performed more frequently in Southern Europe than in Northern Europe in the workup of CSU in children. Overall, 83% of the physicians surveyed reported performing differential blood count, 62% determined thyroid hormones, 59% total IgE, 55% thyroid autoantibodies, 51% antinuclear antibodies (ANA), 50% C‑reactive protein (CRP), 42% screened for parasites [24].
In Iran, intestinal parasitosis was detectable in 4.1% (n = 7) of 169 patients with urticaria compared to 2.9% without (6 of 210) [25]. In a study from Turkey of 211 children with CSU, parasites (mostly Blastocystis hominis) were detectable in 21 (10%) of the children. After antiparasitic therapy, urticaria disappeared in 10 patients, improved in 6, and persisted in 5 [10]. In Italy, 41 of 466 patients with chronic urticaria were sensitized to anisakis, but mostly due to cross-reactive antibodies and primary sensitization to other allergens [26].
In CindU, it is recommended to identify the trigger causing wheals, usually further diagnostic measures are not recommended once the diagnosis is confirmed [2, 3].
For children, the following basic laboratory tests are recommended similar to those for adults: differential blood count, CRP and/or erythrocyte sedimentation rate, thyroid antibodies, and total IgE [2, 3]. From our point of view, it may be advisable to particularly exclude oxyuriasis and, if necessary, other worm diseases (Table 1).
Differential diagnoses to urticaria
Autoinflammatory syndromes are characterized by urticarial skin lesions that accompany general complaints, especially fever and joint issues. In this context, urticarial skin lesions are triggered in particular by cold; if associated with fever episodes, cryopyrin-associated periodic syndromes (CAPS) must be considered. Other symptoms may include arthralgia and bone pain. Persistent inflammation may lead to sensineural hearing loss, musculoskeletal symptoms, chronic aseptic meningitis, and skeletal abnormalities [27]. Despite cold-triggered episodes, the cold test usually yields negative results; elevated inflammatory parameters such as CRP and serum amyloid A are indicative in laboratory results. The diagnosis is confirmed by genetic testing, in which mutations in the NLRP3 gene can be detected, leading to increased interleukin‑1 expression [27]. Therapeutically, IL1 blockade is highly effective, and adequate and early therapy can prevent nonreversible organ damage [28].
Infantile Still’s disease (systemic juvenile idiopathic arthritis [sJIA]), which belongs to the group of juvenile idiopathic arthritis, is characterized by recurrent daily episodes of fever associated with transient, occasionally pruritic exanthema, joint pain, and, as it progresses, arthritis, lymphadenopathy, hepato- and/or splenomegaly, and serositis (pleuritis, pericarditis) [29]. The transient skin lesions resemble urticaria. Again, timely diagnosis and treatment are crucial to prevent subsequent internal organ and joint damage [29]. Joint symptoms present early, arthritis often manifests after several weeks. Laboratory findings include leukocytosis with neutrophilia, CRP and ferritin elevation, and thrombocytosis [30].
If angioedema occurs exclusively, hereditary angioedema must also be ruled out, which frequently manifests for the first time during puberty. A detailed family and medication history are essential. The initial manifestation of the disease in girls/women is often observed in relation to the use of oral contraceptives [31]. Hereditary angioedema in particular might present with life-threatening laryngeal edema; involvement of the gastrointestinal tract with abdominal pain and cramps is also common [19]. Hereditary angioedema must also be considered when angioedema is triggered by trauma, for example, in the course of more intensive dental treatment or a surgical intervention. In complement-related hereditary angioedema (C1-INH-HAE), a decrease in C4, C1-INH function, and mostly also C1-INH concentration proves the diagnosis. The diagnosis of noncomplement-related hereditary angioedema (HAE nC1-INH), which usually occurs in girls/women but is extremely rare overall, can be confirmed by genetic tests (HAE F12, HAE Plg, HAE ANGPT1, HAE KNG1, HAE MYOF, HAE HS3ST6) [32].
Therapy
Therapy of acute urticaria
Treatment of urticaria in both children and adults should be according to current guidelines [2, 3]. For acute urticaria, nonsedating H1 antihistamines, possibly in combination with a short oral glucocorticoid course, are recommended. In a study of 75 patients with acute urticaria, it was shown that the administration of intravenous dexamethasone in addition to antihistamines had no significant effect on urticaria, and the oral administration of prednisolone for 5 days even worsened the outcome compared with patients treated with oral antihistamines alone [33].
Age-specific approvals and child-specific dosages for individual antihistamines must be observed (Table 2). If the effect is not sufficient, it is also recommended to use antihistamines in children in up to 4‑fold dosage (off-label use). The use of first-generation H1 antihistamines is recommended due to adverse effects (anticholinergic effect and sedation) [2, 3].
Therapy of chronic spontaneous urticaria
In step 1, non-sedating H1 antihistamines are recommended in children as well as in adults, if necessary, in increased dosage. Here, too, age-specific approvals and child-specific dosages must be observed (Table 2). Adequate education of patients and parents about off-label use is important. If urticaria is inadequately controlled, omalizumab is recommended add-on to antihistamines in step 2 (Table 3); the anti-IgE antibody is licensed for urticaria from the age of 12 years in a dose of 300 mg subcutaneously every 4 weeks. The current guideline recommends to increase the omalizumab dose in case of insufficient efficacy or to shorten the injection interval (off-label use).
In step 3, ciclosporin A can be administered add-on to antihistamines, but the drug is not approved for the treatment of urticaria (Table 3). In transplant medicine ciclosporin is approved from 1 year of age, but treatment is not recommended in children except for nephrotic syndrome, according to the European Medicines Agency summary of product characteristics (SmPC). One review cites a total of three studies in which a total of 18 children were treated with ciclosporin at doses of 3 and 4 mg/kg bodyweight/day, respectively; all children were symptom-free with ciclosporin [20].
According to the guideline, other immunosuppressants, such as methotrexate and mycophenolate mofetil, may also be used for urticaria, but only small case series have been published; none of these drugs has approval for the treatment of urticaria in children [2, 3].
The guideline also mentions therapy with montelukast; the leukotriene receptor antagonist is approved for the treatment of asthma in patients aged 6 months and older. Individual case reports of efficacy in children are also found in the literature [20, 34]. However, the use of montelukast is limited by neuropsychiatric side effects (including suicidality), and the drug is not approved for urticaria [35].
Oral corticosteroids (OCS) are effective for urticaria but are recommended only for short-term therapy due to the side effects [2, 3]. OCS are recommended in adults for a maximum of 10 days at a dosage of 30–50 mg/day (prednisolone equivalent). In children, the dosage should be adjusted according to age.
Therapy of chronic inducible urticaria
In CIndU, trigger factors should be avoided whenever possible, but it should be noted that trigger avoidance can also decrease children’s quality of life [2, 3].
Nonsedating H1-antihistamines are recommended, in case of nonresponse in up to 4‑fold dosages (off-label use). The efficacy of omalizumab has been shown in the literature for various types of CIndU, but the drug is not approved for this purpose [36]; similar to CSU, omalizumab can be given in higher doses or with a shortened injection interval (up to 600 mg every 2 weeks). The therapeutic goal for CIndU is the complete symptom control [2, 3].
Therapy management
Similar to adults, it is important to adequately document disease severity, disease activity and quality of life in children with urticaria. The UCT (Urticaria Control Test) is available for documentation of urticaria activity, the UAS7 documents the severity of the disease during the last 7 days, and quality of life can be assessed with the CU-Q2oL. For additional or isolated angioedema, disease activity is recorded with the AAS, disease control with the AECT, and quality of life with the AE-QoL [2, 3]. UCT and UAS7 have been validated for childhood [37, 38].
The Children’s Dermatology Life Quality Index (CDLQI) may be helpful in assessing the potential impairment of quality of life, but this has not been developed specifically for urticaria [39].
Course of urticaria in childhood
Infection-associated acute urticaria usually resolves within a few days to weeks, and recurrences are less common as children grow older.
Chronic urticaria in childhood often shows spontaneous recovery, with approximately 40% of children becoming asymptomatic after about three years [9]. A Turkish study showed that 50% of children were symptom-free after five years, with a poorer outcome in girls who developed urticaria after the age of ten [21].
Outlook
At the moment, for the treatment of urticaria in children, nonsedating H1 antihistamines and, for children over the age of twelve, omalizumab are available as approved drugs. Approval of additional drugs that block mast cell mediators, inactivate mast cells, or deplete mast cells are expected [40]. To date, no biomarkers are available to predict the efficacy of a drug. For the selection of drugs, consideration of their impact on comorbidities such as allergic rhinoconjunctivitis, asthma, atopic dermatitis, food allergy, eosinophil esophagitis (EoE), and chronic rhinosinusitis with nasal polyps (CRSwNP) plays a crucial role.
Abbreviations
- ANA:
-
Antinuclear antibodies
- ASST:
-
Autologous serum skin test
- CAPS:
-
Cryopyrin-associated periodic syndrome
- CDLQI:
-
Children’s Dermatology Life Quality Index
- CindU:
-
Inducible urticaria
- CRP:
-
C reactive protein
- CSU:
-
Chronic spontaneous urticaria
- CSUuc:
-
CSU without known cause
- CU:
-
Chronic urticaria
- EAACI:
-
European Academy of Allergy and Clinical Immunology
- NUD:
-
Neutrophilic urticarial dermatosis
- OCS:
-
Oral corticosteroid
- sJIA:
-
Systemic juvenile idiopathic arthritis
- UCT:
-
Urticaria Control Test
References
Shin M, Lee S. Prevalence and causes of childhood urticaria. Allergy Asthma Immunol Res. 2017;9(3):189–90.
Zuberbier T, Altrichter S, Bauer S, Brehler R, Brockow K, Dressler C, et al. S3-Leitlinie Urtikaria. Teil 1: Klassifikation und Diagnostik der Urtikaria – deutschsprachige Adaptation der internationalen S3-Leitlinie. J Dtsch Dermatol Ges. 2023;21(1):81–95. https://doi.org/10.1111/ddg.14906_g.
Zuberbier T, Altrichter S, Bauer S, Brehler R, Brockow K, Dressler C, et al. S3-Leitlinie Urtikaria. Teil 2: Therapie der Urtikaria – deutschsprachige Adaption der internationalen S3-Leitlinie. J Dtsch Dermatol Ges. 2023;21(2):202–16. https://doi.org/10.1111/ddg.14932_g.
Brüske I, Standl M, Weidinger S, Klümper C, Hoffmann B, Schaaf B, et al. Epidemiology of urticaria in infants and young children in Germany—Results from the German LISAplus and GINIplus Birth Cohort Studies. Pediatr Allergy Immunol. 2014;25(1):36–42. https://doi.org/10.1111/pai.12146.
Lee SJ, Ha EK, Jee HM, Lee KS, Lee SW, Kim MA, et al. Prevalence and risk factors of urticaria with a focus on chronic urticaria in children. Allergy Asthma Immunol Res. 2017;9(3):212–9.
Ott H. Chronic urticaria in childhood: Rational diagnostics and treatment. Hautarzt. 2017;68(7):571–82.
Broekaert SMC, Böer-Auer A, Kerl K, Herrgott I, Schulz X, Bonsmann G, et al. Neutrophilic epitheliotropism is a histopathological clue to neutrophilic urticarial dermatosis. Am J Dermatopathol. 2016;38(1):39–49.
Ferrer M. Epidemiology, healthcare, resources, use and clinical features of different types of urticaria. Alergológica 2005. J Investig Allergol Clin Immunol. 2009;19(Suppl 2):21–6.
Wedi B, Raap U, Wieczorek D, Kapp A. Urticaria and infections. Allergy Asthma Clin Immunol. 2009;5(1):1–12.
Yilmaz EA, Karaatmaca B, Sackesen C, Sahiner UM, Cavkaytar O, Sekerel BE, et al. Parasitic infections in children with chronic spontaneous urticaria. Int Arch Allergy Immunol. 2016;171(2):130–5.
Liu TH, Lin YR, Yang KC, Chou CC, Chang YJ, Wu HP. First attack of acute urticaria in pediatric emergency department. Pediatr Neonatol. 2008;49(3):58–64. https://doi.org/10.1016/S1875-9572(08)60014-5.
Huang K, Tao S, Zhou X, Mo J, Zhu B, Shen P, et al. Incidence rates of health outcomes of interest among Chinese children exposed to selected vaccines in Yinzhou Electronic Health Records: A population-based retrospective cohort study. Vaccine. 2020;38(18):3422–8.
Franceschini F, Bottau P, Caimmi S, Cardinale F, Crisafulli G, Liotti L, et al. Evaluating children with suspected allergic reactions to vaccines for infectious diseases. Allergy Asthma Proc. 2018;39(3):177–83.
Wang R, Mathes S, Claussen C, Biedermann T, Brockow K. Cutaneous reactions following COVID-19 vaccination assessed by dermatologists: a single-institutional study in Germany. J Dtsch Dermatol Ges. 2023;21(3):255–62.
Prasad S, McMahon DE, Tyagi A, Ali R, Singh R, Rosenbach M, et al. Cutaneous reactions following booster dose administration of COVID-19 mRNA vaccine: A first look from the American Academy of Dermatology/International League of Dermatologic Societies registry. JAAD Int. 2022;8:49–51. https://doi.org/10.1016/j.jdin.2022.04.004.
Brockow K, Wang R, Mathes S, Bent R, Faihs V. SARS-CoV‑2 and allergy—what have we learned after two and a half years ? Allergol Select. 2023;7:101–12.
Ring J, Beyer K, Biedermann T, Bircher A, Fischer M, Fuchs T, et al. Leitlinie zu Akuttherapie und Management der Anaphylaxie – Update 2021. AL. 2021;44(5):356–88.
Kolkhir P, Muñoz M, Asero R, Ferrer M, Kocatürk E, Metz M, et al. Autoimmune chronic spontaneous urticaria. J Allergy Clin Immunol. 2022;149(6):1819–31.
Ocak M, Nain E, Şahiner ÜM, Akin MŞ, Karabiber E, Şekerel BE, et al. Recurrent angioedema in childhood: hereditary angioedema or histaminergic angioedema? Pediatr Dermatol. 2021;38(1):143–8.
Cornillier H, Giraudeau B, Munck S, Hacard F, Jonville-Bera AP, d’Acremont G, et al. Chronic spontaneous urticaria in children—a systematic review on interventions and comorbidities. Pediatr Allergy Immunol. 2018;29(3):303–10.
Sahiner UM, Tuncer A, Tolga S. Chronic urticaria : etiology and natural course in children. Int Arch Allergy Immunol. 2011;156(2):224–30.
Caminiti L, Passalacqua G, Magazzu G, Comisi F, Vita D, Barberio G, et al. Chronic urticaria and associated coeliac disease in children: a case-control study. Pediatr Allergy Immunol. 2005;16(5):428–32. https://doi.org/10.1111/j.1399-3038.2005.00309.x.
Ludvigsson JF, Lindelöf B, Rashtak S, Rubio-Tapia A, Murray JA. Does urticaria risk increase in patients with celiac disease? A large population-based cohort study. Eur J Dermatol. 2013;23(5):681–7.
Tsabouri S, Arasi S, Beken B, Church MK, Alvaro-Lozano M, Caffarelli C, et al. A European survey of management approaches in chronic urticaria in children: EAACI pediatric urticaria taskforce. Pediatr Allergy Immunol. 2022;33(1):e13674. https://doi.org/10.1111/pai.13674.
Fakhar M, Ghaffari J, Dabbaghzadeh A, Charati JY, Ghaffari B, Esboei BR. Prevalence of intestinal parasites among patients with chronic urticaria in northern Iran. Infect Disord Drug Targets. 2021;21(1):130–3. https://www.eurekaselect.com/181420/article.
Brusca I, Graci S, Barrale M, Cammilleri G, Zarcone M, Onida R, et al. Use of a comprehensive diagnostic algorithm for Anisakis allergy in a high seroprevalence Mediterranean setting. Eur Ann Allergy Clin Immunol. 2020;52(03):74.
Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, Kone-Paut I, Goldbach-Mansky R, Lachmann H, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017;76(6):942–7. https://doi.org/10.1136/annrheumdis-2016-209686.
Ostring GT, Singh-Grewal D. Periodic fevers and autoinflammatory syndromes in childhood. J Paediatr Child Health. 2016;52(9):865–71.
Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, et al. Toward new classification criteria for juvenile idiopathic arthritis: First steps, pediatric rheumatology international trials organization international consensus. J Rheumatol. 2019;46(2):190–7.
Zaripova LN, Midgley A, Christmas SE, Beresford MW, Baildam EM, Oldershaw RA. Juvenile idiopathic arthritis: from aetiopathogenesis to therapeutic approaches. Pediatr Rheumatol. 2021;19(1):1–14.
Agostoni A, Aygören-Pürsün E, Binkley KE, Blanch A, Bork K, Bouillet L, et al. Hereditary and acquired angioedema: Problems and progress: Proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004;114(3 Suppl):S51. https://doi.org/10.1016/j.jaci.2004.06.047.
Bork K, Wulff K, Witzke G, Staubach P, Hardt J, Meinke P. Gene mutations linked to hereditary angioedema in solitary Angioedema patients with normal C1 inhibitor. J Allergy Clin Immunol Pract. 2023; https://doi.org/10.1016/j.jaip.2023.01.051.
Palungwachira P, Vilaisri K, Musikatavorn K, Wongpiyabovorn J. A randomized controlled trial of adding intravenous corticosteroids to H1 antihistamines in patients with acute urticaria. Am J Emerg Med. 2021;42:192–7.
Sanada S, Tanaka T, Kameyoshi Y, Hide M. The effectiveness of montelukast for the treatment of anti-histamine-resistant chronic urticaria. Arch Dermatol Res. 2005;297(3):134–8. https://doi.org/10.1007/s00403-005-0586-4.
Drug Safety Mail 2019-59 Arzneimittelkommission der deutschen Ärzteschaft (AkdÄ) vom 30.10.2019.. https://www.akdae.de/arzneimittelsicherheit/drug-safety-mail/newsdetail/drug-safety-mail-2019-59. Accessed 26 June 2023.
Maurer M, Metz M, Brehler R, Hillen U, Jakob T, Mahler V, et al. Omalizumab treatment in patients with chronic inducible urticaria: A systematic review of published evidence. J Allergy Clin Immunol. 2018;141(2):638–49.
Prosty C, Gabrielli S, Mule P, Noorah N, Baum S, Greenberger S, et al. Validation of the urticaria control test (UCT) in children with chronic urticaria. J Allergy Clin Immunol Pract. 2022;10(12):3293–3298.e2.
Gabrielli S, Mulé P, Prosty C, Gooding G, Le M, Zhang L, et al. Validation of UAS7 among children with chronic spontaneous urticaria. J Allergy Clin Immunol Pract. 2022;10(7):1927–1929.e1.
Loo WJ, Diba V, Chawla M, Finlay AY. Epidemiology and health services research dermatology life quality index : influence of an illustrated version. Br J Dermatol. 2003;148(2):279–84
Kolkhir P, Elieh-Ali-Komi D, Metz M, Siebenhaar F, Maurer M. Understanding human mast cells: lesson from therapies for allergic and non-allergic diseases. Nat Rev Immunol. 2022;22(5):294–308.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.-C.E. Brehler declares that there are no conflicts of interest. A. Bauer is or has been a presenter and/or advisor and/or has received research support from Novartis, Genentec, Leo, Sanofi, Regeneron, Shire, Takeda, Amgen, AstraZeneca, Abbvie, Celldex, Lilly, Pharvaris, Almirall, L’Oreal, Incyte, Blueberry Therapeutics, Centogene, Galderma and Biofrontera. B. Wedi has received honoraria for lectures and multihour advisory boards, respectively, as well as funding for travel/congress attendance in the last three years from: ALK-Abelló, Bencard, Biocryst, CSL Behring, HAL Allergy, Kalvista, Novartis, Takeda and ThermoFisher Scientific.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brehler, AC.E., Bauer, A. & Wedi, B. Urticaria in childhood—what’s new?. Allergo J Int 32, 318–325 (2023). https://doi.org/10.1007/s40629-023-00271-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-023-00271-8