Abstract
Background and Objective
Sepsis and continuous renal replacement therapy (CRRT) can both significantly affect antifungal pharmacokinetics. This study aimed to describe the pharmacokinetics of caspofungin in critically ill patients during different CRRT modes.
Methods
Patients receiving caspofungin and undergoing continuous veno-venous haemofiltration (CVVH) or haemodiafiltration (CVVHDF) were eligible to take part in the study. Blood samples were collected at seven sampling times during a dosing interval. Demographics and clinical data were recorded. Population pharmacokinetic analysis and Monte-Carlo simulation were undertaken using Pmetrics.
Results
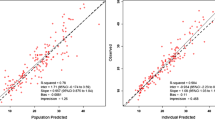
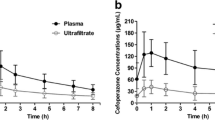
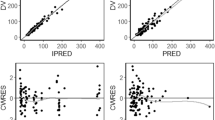
Twelve pharmacokinetic profiles from nine patients were analysed. The caspofungin CRRT clearance (CL) was 0.048 ± 0.12 L/h for CVVH and 0.042 ± 0.042 L/h for CVVHDF. A two-compartment linear model best described the data. Patient weight was the only covariate affecting drug CL and central volume. The mean (standard deviation) parameter estimates were 0.64 ± 0.12 L/h for CL, 9.35 ± 3.56 L for central volume, 0.25 ± 0.19 per h for the rate constant for drug distribution from central to peripheral compartments and 0.19 ± 0.10 per h from peripheral to central compartments. Based on simulation results, a caspofungin 100 mg loading dose followed by a 50 mg maintenance dose for patients with a total body weight of ≤80 kg best achieved the pharmacokinetic/PD targets whilst a 70 mg maintenance dose was required for patients with a weight of >80 kg.
Conclusion
No caspofungin dosing adjustment is necessary for patients undergoing either form of CRRT. However, higher than recommended loading doses of caspofungin are required to achieve pharmacokinetic/pharmacodynamic targets in critically ill patients.
Registration: ClinicalTrials.gov Identifier NCT01403220




Similar content being viewed by others
References
Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–9.
Grim SA, Berger K, Teng C, Gupta S, Layden JE, Janda WM, et al. Timing of susceptibility-based antifungal drug administration in patients with Candida bloodstream infection: correlation with outcomes. J Antimicrob Chemother. 2012;67(3):707–14.
Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54(12):1739–46.
Bassetti M, Righi E, Ansaldi F, Merelli M, Trucchi C, De Pascale G, et al. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med. 2014;40(6):839–45.
Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37(3):840–51 (quiz 59).
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8.
Friedrich JO, Wald R, Bagshaw SM, Burns KE, Adhikari NK. Hemofiltration compared to hemodialysis for acute kidney injury: systematic review and meta-analysis. Crit Care. 2012;16(4):R146.
Pea F, Viale P, Pavan F, Furlanut M. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin Pharmacokinet. 2007;46(12):997–1038.
Choi G, Gomersall CD, Tian Q, Joynt GM, Freebairn R, Lipman J. Principles of antibacterial dosing in continuous renal replacement therapy. Crit Care Med. 2009;37(7):2268–82.
Roger C, Wallis SC, Louart B, Lefrant JY, Lipman J, Muller L, et al. Comparison of equal doses of continuous venovenous haemofiltration and haemodiafiltration on ciprofloxacin population pharmacokinetics in critically ill patients. J Antimicrob Chemother. 2016;71(6):1643–50.
Leitner JM, Meyer B, Fuhrmann V, Saria K, Zuba C, Jager W, et al. Multiple-dose pharmacokinetics of anidulafungin during continuous venovenous haemofiltration. J Antimicrob Chemother. 2011;66(4):880–4.
Martial LC, Bruggemann RJ, Schouten JA, van Leeuwen HJ, van Zanten AR, de Lange DW, et al. Dose reduction of caspofungin in intensive care unit patients with Child Pugh B will result in suboptimal exposure. Clin Pharmacokinet. 2016;55(6):723–33. doi:10.1007/s40262-015-0347-2.
Muilwijk EW, Schouten JA, van Leeuwen HJ, van Zanten AR, de Lange DW, Colbers A, et al. Pharmacokinetics of caspofungin in ICU patients. J Antimicrob Chemother. 2014;69(12):3294–9.
Yang Q, Wang T, Xie J, Wang Y, Zheng X, Chen L, et al. Pharmacokinetic/pharmacodynamic adequacy of echinocandins against Candida spp. in intensive care unit patients and general patient populations. Int J Antimicrob Agents. 2016;47(5):397–402.
Sinnollareddy MG, Roberts JA, Lipman J, Akova M, Bassetti M, De Waele JJ, et al. Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: data from multinational Defining Antibiotic Levels in Intensive care unit (DALI) patients study. Crit Care. 2015;19:33.
European Medicines Agency. Cancidas: EPAR. Last updated 1 Jun 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000379/WC500021033.pdf. Accessed 8 July 2016.
Pound MW, Townsend ML, Drew RH. Echinocandin pharmacodynamics: review and clinical implications. J Antimicrob Chemother. 2010;65(6):1108–18.
Weiler S, Seger C, Pfisterer H, Stienecke E, Stippler F, Welte R, et al. Pharmacokinetics of caspofungin in critically ill patients on continuous renal replacement therapy. Antimicrob Agents Chemother. 2013;57(8):4053–7.
Joannidis M, Oudemans-van Straaten HM. Clinical review: patency of the circuit in continuous renal replacement therapy. Crit Care. 2007;11(4):218.
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63.
Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–800.
Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012;34(4):467–76.
Tatarinova T, Neely M, Bartroff J, van Guilder M, Yamada W, Bayard D, et al. Two general methods for population pharmacokinetic modeling: non-parametric adaptive grid and non-parametric Bayesian. J Pharmacokinet Pharmacodyn. 2013;40(2):189–99.
Mentre F, Escolano S. Prediction discrepancies for the evaluation of nonlinear mixed-effects models. J Pharmacokinet Pharmacodyn. 2006;33(3):345–67.
Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, Lepak A. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob Agents Chemother. 2010;54(6):2497–506.
Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol. 2013;51(8):2571–81.
Gonzalez de Molina F, Martinez-Alberici Mde L, Ferrer R. Treatment with echinocandins during continuous renal replacement therapy. Crit Care. 2014;18(2):218.
Stone JA, Xu X, Winchell GA, Deutsch PJ, Pearson PG, Migoya EM, et al. Disposition of caspofungin: role of distribution in determining pharmacokinetics in plasma. Antimicrob Agents Chemother. 2004;48(3):815–23.
Wurthwein G, Cornely OA, Trame MN, Vehreschild JJ, Vehreschild MJ, Farowski F, et al. Population pharmacokinetics of escalating doses of caspofungin in a phase II study of patients with invasive aspergillosis. Antimicrob Agents Chemother. 2013;57(4):1664–71.
Betts RF, Nucci M, Talwar D, Gareca M, Queiroz-Telles F, Bedimo RJ, et al. A Multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis. 2009;48(12):1676–84.
Migoya EM, Mistry GC, Stone JA, Comisar W, Sun P, Norcross A, et al. Safety and pharmacokinetics of higher doses of caspofungin in healthy adult participants. J Clin Pharmacol. 2011;51(2):202–11.
Louie A, Deziel M, Liu W, Drusano MF, Gumbo T, Drusano GL. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob Agents Chemother. 2005;49(12):5058–68.
Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, et al. Interlaboratory variability of Caspofungin MICs for Candida spp. Using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother. 2013;57(12):5836–42.
Nguyen TH, Hoppe-Tichy T, Geiss HK, Rastall AC, Swoboda S, Schmidt J, et al. Factors influencing caspofungin plasma concentrations in patients of a surgical intensive care unit. J Antimicrob Chemother. 2007;60(1):100–6.
Acknowledgements
The authors thank Loubna Elotmani, Audrey Ayral, Sophie Lloret, Jenny Ordonez and all ICU nurses for their help in sampling, collecting data and assay.
Authors’ contributions
CR made substantial contributions to conception, study design, acquisition and interpretation of data, drafting of the manuscript and approved the final version to be published. SCW made substantial contributions to acquisition, analysis and interpretation of data, revision of the manuscript for important intellectual content and approved the final version to be published. LM made substantial contributions to conception, study design, interpretation of data, drafting of the manuscript and approved the final version to be published. GS made substantial contributions to acquisition, analysis and interpretation of data, revision of the manuscript for important intellectual content and approved the final version to be published. JL made substantial contributions to conception, study design, interpretation of data, revision of the manuscript for important intellectual content and approved the final version to be published. RB made substantial contributions to interpretation of data, drafting of the manuscript and approved the final version to be published. JY made substantial contributions to conception, study design, interpretation of data, drafting of the manuscript and approved the final version to be published. JAR made substantial contributions to conception, study design, analysis and interpretation of data, drafting of the manuscript, revision of the manuscript for important intellectual content and approved the final version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the local ethics committee in Nîmes, France (Comité de Protection des Personnes Sud Mediterranée III 2012.02.05). Written informed consent was obtained from either the patient or their nominated substitute decision maker.
Conflict of interest
JAR is funded by a Career Development Fellowship from the National Health and Medical Research Council of Australia (APP1048652). The authors would like to acknowledge other funding to the Burns Trauma Critical Care Research Centre from the National Health and Medical Research Council of Australia for a Project Grant (APP1044941) and Centre for Research Excellence (APP1099452). RJB declares that he has served as a consultant to and has received unrestricted and research grants from Astellas Pharma Inc., Gilead Sciences, Merck Sharpe and Dohme Corp., and Pfizer Inc. All payments were invoiced by the Radboud University Medical Center. CR, SW, LM, GS, JYL, JL have no conflict of interest to declare.
Funding
This work was supported by academic Grant from the Nîmes University Hospital.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roger, C., Wallis, S.C., Muller, L. et al. Caspofungin Population Pharmacokinetics in Critically Ill Patients Undergoing Continuous Veno-Venous Haemofiltration or Haemodiafiltration. Clin Pharmacokinet 56, 1057–1068 (2017). https://doi.org/10.1007/s40262-016-0495-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0495-z