Abstract
Background and Objectives
Ropanicant hydrochloride (previously known as SUVN-911, hereinafter referred to as ropanicant) is a novel alpha4 beta2 nicotinic acetylcholine receptor (α4β2 nAchR) antagonist being developed for the treatment of major depressive disorder. The objectives of the present studies were to evaluate the safety, tolerability, and pharmacokinetics of ropanicant after single and multiple ascending doses and to evaluate the effect of food, sex, and age on its pharmacokinetics in healthy subjects.
Methods
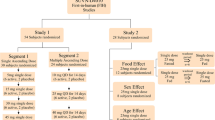
Two phase I studies have been conducted for ropanicant. Study 1 is a randomized, double-blind, placebo-controlled, first-in-human study to evaluate the safety, tolerability, and pharmacokinetics of single ascending doses (0.5, 6, 15, 30, and 60 mg) and multiple ascending doses (15, 30, and 45 mg) of ropanicant administered orally for 14 days to healthy male subjects. In Study 2, the effect of food, sex, and age on ropanicant pharmacokinetics was evaluated following a single 30-mg oral dose.
Results
Ropanicant at single doses up to 60 mg and multiple doses up to 45 mg once daily was found to be safe and well tolerated in healthy subjects. The most frequently reported adverse events were headache and nausea. Ropanicant exposures were more than dose proportional following single and multiple administrations. Urinary excretion of unchanged ropanicant was low across the doses. Upon multiple dosing, 1.5- to 2.5-fold higher exposures for maximum concentration and 1.6- to 4.0-fold higher exposures for area under the concentration–time curve from time 0–24 h were observed on day 14 as compared with day 1. Sex had an effect on the pharmacokinetics of ropanicant as a 64% higher area under the concentration–time curve from time 0 to 24 h and a 26% higher maximum concentration was observed in female adults when compared with male adults. Plasma exposures were comparable in fasted versus fed conditions and in adult versus elderly subjects.
Conclusions
Ropanicant was found to be safe and well tolerated following single and multiple oral administrations in healthy subjects. Ropanicant showed nonlinear pharmacokinetics and accumulation following multiple dosing. Urinary excretion represents an insignificant elimination pathway for ropanicant. Ropanicant pharmacokinetics were sex dependent, and food and age had no effect on its pharmacokinetics.
Clinical Trial Registration
NCT03155503 and NCT03551288.





Similar content being viewed by others
References
Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the Global Burden of Disease study. J Psychiatr Res. 2020;126:134–40.
Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–312.
Kuehner C. Why is depression more common among women than among men? Lancet Psychiatry. 2017;4(2):146–58.
WHO. Depression; 2020. https://www.WHO.int/news-room/fact-sheets/detail/Depression. Accessed 8 Aug 2022.
Ringdal C, Rootjes F. Depression and labor supply: evidence from the Netherlands. Econ Hum Biol. 2022;45: 101103. https://doi.org/10.1016/j.ehb.2021.101103.
Philip NS, Carpenter LL, Tyrka AR, Price LH. Nicotinic acetylcholine receptors and depression: a review of the preclinical and clinical literature. Psychopharmacology. 2010;212:1–12.
Nirogi R, Abraham R, Jayarajan P, Goura V, Kallepalli R, Medapati RB, et al. Ropanicant (SUVN-911), an α4β2 nicotinic acetylcholine receptor antagonist intended for the treatment of depressive disorders: pharmacological, behavioral, and neurochemical characterization. Psychopharmacology. 2022. https://doi.org/10.1007/s00213-022-06108-6.
Nirogi R, Mohammed AR, Shinde AK, Ravella SR, Bogaraju N, Subramanian R, et al. Discovery and development of 3-(6-chloropyridine-3-yloxymethyl)-2-azabicyclo[3.1.0]hexane hydrochloride (SUVN-911): a novel, potent, selective, and orally active neuronal nicotinic acetylcholine α4β2 receptor antagonist for the treatment of depression. J Med Chem. 2020;63(6):2833–53.
International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use.. ICH harmonized tripartite guideline: Guideline for Good Clinical Practice. J Postgrad Med. 2001;47(1):45–50
US Food and Drug Administration. Guidance for industry: bioanalytical method validation. Rockville (MD): Food and Drug Administration; 2018. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf. Accessed 25 May 2022.
US Food and Drug Administration. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Rockville (MD): Food and Drug Administration; 2005. https://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf. Accessed 25 May 2022.
Acknowledgments
The authors acknowledge the support received from Venkat Jasti, the CEO of Suven Life Sciences Ltd. The authors acknowledge the principal investigators and biostatistician from IQVIA Phase One Services (Overland Park, KS, USA) for their support in the conduct of the studies reported in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Suven Life Sciences Ltd.
Conflict of interest
R. Nirogi, V. Benade, V.K. Goyal, S.K. Pandey, A.R. Mohammed, A. Shinde, D. Dogiparti, J. Ravula, S. Jetta, and V.R.C. Palacharla are employees of Suven Life Sciences Ltd.
Ethics approval
The study was conducted in compliance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice Guidelines, and the Declaration of Helsinki. The protocols were reviewed and approved by the institutional review board. The study was conducted at IQVIA Phase One Services, Overland Park, KS, USA.
Consent to participate
All subjects provided written informed consent.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
All authors were involved in the study design and the discussion and interpretation of the results. All authors repeatedly edited the manuscript and approved the final version.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nirogi, R., Benade, V., Goyal, V.K. et al. Safety, Tolerability, and Pharmacokinetics of Ropanicant (SUVN-911), a Novel Alpha4 Beta2 Nicotinic Acetylcholine Receptor (α4β2 nAChR) Antagonist, in Healthy Adult and Elderly Subjects. Clin Drug Investig 42, 747–762 (2022). https://doi.org/10.1007/s40261-022-01189-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01189-9