Abstract
Purpose
We compared the clinical effects of Neurotropin, limaprost alfadex, and a combination of both drugs for lumbar spinal stenosis (LSS) with low back pain (LBP).
Methods
We conducted a multicenter, randomized, active-controlled, open-label trial from March 2021 to May 2022. Participants were patients diagnosed with LSS by MRI and were randomly assigned to three groups: Neurotropin/limaprost combination (NL group), Neurotropin (N group), and limaprost group (L group). Participants received the drugs administered orally for 12 weeks, and each examination and observation was performed before any drug administration and every 2 weeks thereafter. We recorded age, sex, height, weight, duration of symptoms, intermittent claudication distance, level of stenosis in MRI, and concomitant analgesics as examination items in the trial period. Items measured during the trial were visual analog scale (VAS) score (mm) for LBP, leg pain and numbness, walking activity (walking speed, stride length), standing balance (3 m Timed Up-and-Go (TUG) Test results, Five Times Sit-to-Stand Test (FTSST) results), LBP/Quality of Life (QOL)-related scores (Oswestry Disability Index (ODI), Euro QOL 5-Dimensions 5-Level (EQ-5D-5L), Roland–Morris Disability Questionnaire (RDQ)), psychological factors (Pain catastrophizing scale (PCS) and Pain Self-Efficacy Questionnaire (PSEQ) scores), and adverse events. Each item was evaluated using changes at each visit (weeks 2–12) from baseline value before drug administration (week 0), and changes were considered significant when p < 0.05.
Results
We included results from 64 patients in the present study; 24 were assigned to the NL group (mean age 71.2 years), 20 to the N group (mean age 76.2 years), and 20 to the L group (mean age 74.4 years). There were no significant differences between the three groups in patient characteristics, concomitant analgesics, or baseline VAS score, gait balance, or QOL-related scores (p ≥ 0.05). The VAS and leg pain scores were significantly improved in Group L, and LBP was improved significantly in Group N. QOL and ODI scores improved significantly in the NL and L groups, EQ-5D score improved significantly in the L group, and RDQ score improved significantly in all groups (p < 0.05). Psychological factor and PCS scores improved significantly in the NL and L groups (p < 0.05). Walking speed and stride length were improved significantly in the NL and N groups (p < 0.05). TUG/FTSST scores were improved significantly in all groups (p < 0.05).
Leg pain VAS score was improved significantly (p < 0.05) in the L group compared with the NL group after 6 and 12 weeks of administration, and LBP VAS was improved significantly in the N group after 6 weeks compared with the NL group (p < 0.05). Walking speed was significantly improved in the NL group after 2 weeks compared with the N group and improved significantly in the NL group after 6 weeks (p < 0.05) compared with the L group. RDQ was decreased significantly in the L group compared with the NL group after 8 weeks (p < 0.05).
Conclusions
Combined use of Neurotropin and limaprost showed an additional effect on walking speed compared with single drug administration. Neurotropin may contribute to the improvement of low back pain, walking speed/stride length, and standing balance.
Trial Registration
Japan Registry of Clinical Trials (jRCTs031200282).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Neurotropin is a nonprotein extract from inflamed skin tissue of rabbits inoculated with the vaccinia virus and is widely used in Japan to treat neuropathic pain such as postherpetic neuralgia and other painful conditions. Neurotropin does not inhibit prostaglandin biosynthesis, side effects such as gastrointestinal disorders are less than those of nonsteroidal anti-inflammatory drugs (NSAIDs), and it has the advantage of being able to be administered for a long time. |
Because Neurotropin relieves neuropathic pain, it is expected to be effective for lumbar spinal stenosis (LSS), but there is no research on the effect of Neurotropin on LSS. |
We conducted a multicenter, randomized, active-controlled, open-label study to investigate the effects of Neurotropin, limaprost, and combination therapy on clinical symptoms, Quality of Life (QOL)-related scores (Oswestry Disability Index (ODI), Euro QOL 5-Dimensions 5-Level (EQ-5D-5L), Roland–Morris Disability Questionnaire (RDQ), psychological factors, walking activity, and standing balance in patients with LSS and low back pain (LBP). |
What was learned from the study? |
Neurotropin contributed to the improvement of LBP, walking activity, and standing balance. |
By contrast, limaprost contributed to the improvement of leg pain, QOL-related scores, and standing balance. |
Combined use of Neurotropin and limaprost showed an additional effect on walking speed compared to single drug administration. |
Introduction
The concept of lumbar spinal stenosis (LSS) has become widely known since it was reported in 1954 by Verbiest [1]. In 1976, Arnoldi et al. [2] proposed an international definition and classification of LSS that is still used widely today. LSS is a narrowing of the spinal canal or intervertebral foramen of the lumbar spine, resulting in damage to nerve tissue or blood flow, with consequent clinical symptoms of neurogenic claudication. It often causes low back pain (LBP), leg pain, gait disturbance, and other disabilities in the elderly [3]. LSS affects approximately 103 million people worldwide and affects 11% of older adults in the United States [4]. In a Japanese cohort study [5] with an average age of 66, the prevalence of symptomatic LSS was approximately 10%. The prevalence of LSS increases in both men and women after the age of 50, and the number of patients with LSS is expected to increase in the future as the population ages. Diagnosis can be based on clinical manifestations, such as intermittent claudication, and imaging findings, such as computed tomography and magnet resonance imaging (MRI). Conservative treatment, such as drug and physical therapies, is performed as the first choice.
Pain is usually managed with analgesics, such as acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). Mild narcotic analgesics are indicated if the pain is intractable and unresponsive to or intolerable with other analgesics [4].
Radiculopathy associated with degenerative disease of the lumbar spine has been reported to result from a combination of mechanical nerve root compression and consequent circulatory disturbances. Impaired blood flow in the cauda equina and nerve roots plays an important role in the mechanism of intermittent claudication in patients with LSS [6]. Prostaglandin E1 (PGE 1) is a potent vasodilator and inhibitor of platelet aggregation and is of interest as a therapeutic agent for LSS with intermittent claudication. The prostaglandin E1 derivative, limaprost alfadex, improves leg pain, leg numbness, and intermittent claudication in patients with LSS [7,8,9]. However, the use of limaprost has limitations, such as the requirement for careful administration to patients with a bleeding tendency and patients receiving antiplatelet agents, thrombolytic agents, or anticoagulants, making it difficult to use limaprost in such patients.
By contrast, Neurotropin is a nonprotein extract from inflamed skin tissue of rabbits inoculated with the vaccinia virus and is widely used in Japan to treat neuropathic pain such as postherpetic neuralgia and other painful conditions. Unlike NSAIDs and opioids, Neurotropin does not act on prostaglandin production or opioid systems, but activates the descending pain suppression system via monoamines in the brain and regulates autonomic nerves, resulting in a reduction of neuropathic pain [10, 11]. Neurotropin has no serious side effects and is well tolerated so it can be used even in patients receiving antiplatelet drugs. In addition, since Neurotropin does not inhibit prostaglandin biosynthesis, side effects such as gastrointestinal disorders are less than those of NSAIDs, and it has the advantage of being able to be administered for a long time. Because Neurotropin relieves neuropathic pain, it is expected to be effective for LSS, but there is no research on the effect of Neurotropin on LSS.
To examine and compare the clinical effects of Neurotropin on LSS with low back pain, participants in the present study were divided into three treatment groups: Neurotropin, limaprost, and both drugs in an open-label manner, and we compared the efficacy of each drug, and the additional effect of concomitant Neurotropin use.
Methods
Trial Design
This study was conducted from March 2021 to May 2022 as a multicenter, randomized, active-controlled, open-label study at six research centers in Japan (Chiba University Hospital, Chiba Qiball Clinic, Shimoshizu National Hospital, Eastern Chiba Medical Center, JCHO Funabashi Central Hospital, and Konodai Hospital). The study received approval from the Clinical Research Review Committee, Chiba University Certified Review Board (CRB No. CRB0043-20), which notified all participating centers of the approval. This study was registered at the Japan Registry of Clinical Trials under the identifier jRCTs031200282. The study was conducted in accordance with the ethical principles, clinical research laws, and relevant notifications stipulated in the Declaration of Helsinki (as revised in 2013). Study protocols were reviewed and approved by the ethics committees of all study sites. All participants provided documented written informed consent before study procedures were initiated. Physicians obtained documented consent to participate in the study from patients who met the inclusion criteria and did not meet the exclusion criteria and enrolled the patient participants in the data center for this study. Enrolled patients were randomly assigned to three groups: a Neurotropin/limaprost combination group (NL group), a Neurotropin group (N group), and a limaprost group (L group), using a stratified permuted block method, and gender was set as an allocation adjustment factor. The participants took their assigned study drug(s) for 12 weeks.
Patients
In group N, 2 tablets of 4 units of Neurotropin were administered orally twice a day. In group L, 1 tablet of limaprost (5 μg) was administered orally 3 times a day. In the NL group, 2 tablets of 4 units of Neurotropin were administered twice daily, and 1 tablet of limaprost (5 μg) was administered 3 times daily. After two visits (Visits 1 and 2) for screening and to obtain written informed consent to participate in the study, the patients visited the hospital 7 times (Visit 3 (0 w) to Visit 9 (12 w)) every 2 weeks during the study drug administration period, and the various items were measured.
Inclusion criteria were as follows: (1) patients who gave documented written informed consent for this study, (2) patients aged >20 years, (3) patients with LBP (VAS score >30 mm), (4) patients with LSS on MR imaging, (5) patients with intermittent claudication, (6) patients who have not taken Neurotropin or limaprost within the 12 weeks before their consent to participate in the trial, and (7) patients who have not taken prostaglandin preparations within 7 days before their consent to participate in the trial. Exclusion criteria were (1) patients with a history of lumbar surgery, (2) patients scheduled for surgery for LSS, (3) patients with lumbar disc herniation, (4) patients with arteriosclerosis obliterans, (5) patients with diabetes or alcoholic peripheral neuropathy, (6) patients who underwent nerve root block within 3 months of their consent to participate in the trial, (7) patients with paralysis due to cerebral hemorrhage or cerebral infarction, and (8) patients with a severe respiratory, heart, renal, or liver disease, or blood disorder. In principle, the analgesics and rehabilitation treatments that had been used before participating in the present study could be continued.
Evaluation item
Age, sex, height, weight, duration of symptoms, intermittent claudication distance, level of stenosis on MRI, and concomitant analgesics were investigated as endpoints during the trial. Items measured during the trial were visual analog scale (VAS) score (mm) (0 mm, no pain; 10 mm, worst pain ever experienced) for LBP, leg pain, and numbness, walking activity (walking speed, stride length), standing balance (3 m Timed Up-and-Go (TUG) test results, Five Times Sit-to-Stand Test (FTSST) results), LBP/Quality of Life (QOL) scores (Oswestry Disability Index (ODI), Euro QOL 5-Dimensions 5-Level (EQ-5D-5L), Roland–Morris Disability Questionnaire (RDQ)), psychological factors (Pain Catastrophizing Scale (PCS) and Pain Self-Efficacy Questionnaire (PSEQ) scores), and adverse events. Each item was evaluated using changes at each visit (weeks 2–12) from baseline value before drug administration (week 0), and changes were considered significant when p < 0.05. Participants used a measurement application that utilizes the GPS function of an iPhone 7 (iOS13) at each visit from Visit 3 to 9, and measured walking speed (m), stride length (m), and TUG test (min) by walking for 20 s, and FTSST (min).
Statistical analysis
For patient backgrounds and concomitant analgesics in each randomized group, analysis of variance (ANOVA) for continuous variables and a Fisher exact test for categorical variables were used to confirm the presence or absence of bias between groups.
Using the amount of change from baseline (Visit 3, 0 w) at each visit from Visit 4 (2 w) to Visit 9 (12 w) as the efficacy endpoint, a mixed-effects model for repeated measures (MMRM) was used to calculate the estimated difference between groups, its 95% confidence interval, and p.
The MMRM included baseline values for each endpoint, group, Visit, group-Visit interactions, sex (randomization adjustment factor) as fixed effects, and patient as a random effect.
All data at the baseline are expressed as the mean ± standard deviation (SD). We considered differences with p < 0.05 as significant. Statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA).
Results
Patients
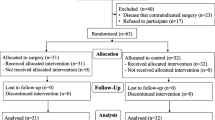
We enrolled 66 patients in the present study and randomly assigned 24 to the NL group, 22 to the N group, and 20 to the L group. Two patients in the N group were excluded because they had not taken the drug, and ultimately data from 24 patients in the NL group, 20 in the N group, and 20 in the L group were included in the present analysis (Fig. 1).
There were no significant differences between the three groups in the baseline values for patient background, VAS score, LBP/QOL score, psychological factors, walking activity, and standing balance (p ≥ 0.05) (Tables 1 and 2). There was no significant difference between the three groups for concomitant analgesics (p ≥ 0.05) (Table 3).
-
(1)
Intragroup comparison (change from baseline) (Tables 4 and 5).
-
•
Leg pain VAS values improved significantly after 12 weeks (–11.3 mm [p = 0.031]) in the L group.
-
•
The LBP VAS value improved significantly after 4 weeks (–9.3 mm [p = 0.022]) and 6 weeks (–8.4 mm [p = 0.041]) in the N group.
-
•
The ODI score improved significantly after 12 weeks (–4.7% [p = 0.028]) in the NL group. In the L group, the ODI score was improved significantly after 4 weeks (–4.6% [p = 0.016]), 8 weeks (–5.0% [p = 0.026]), and 12 weeks (–4.6% [p = 0.026]). 0.043]).
-
•
The EQ-5D-5L score improved significantly in the L group after 4 weeks (0.068 [p = 0.004]).
-
•
The RDQ score improved significantly after 12 weeks (–1.5 [p = 0.024]) in the NL group. The RDQ score significantly improved after 8 weeks (–1.5 [p = 0.048]) and 12 weeks (–1.5 [p = 0.034]) in the N group. In addition, the RDQ score in the L group was significantly improved after 4 weeks (–1.4 [p = 0.048]), 8 weeks (–2.3 [p = 0.001]), and 12 weeks (–1.8 [p = 0.009]).
-
•
The PCS score improved significantly after 8 weeks (–5.4 [p = 0.004]) in the NL group. The PCS score improved significantly after 4 weeks (–3.8 [p = 0.038]) in the L group.
-
•
None of the drugs significantly improved the PSEQ score.
-
•
Walking speed improved significantly after 2 weeks (0.13 m/s [p = 0.027]), 4 weeks (0.08 m/s [p = 0.043]), 6 weeks (0.16 m/s [p = 0.015]), and 12 weeks (0.21 m/s [p = 0.001]) in the NL group. Walking speed improved significantly after 4 weeks (0.16 m/s [p = 0.002]) in the N group.
-
•
Stride length improved significantly after 12 weeks (0.05 m [p = 0.015]) in the NL group and after 4 weeks (0.03 m [p = 0.015]) in the N group.
-
•
TUG test improved significantly after 4 weeks (–1.33 s [p = 0.005]), 6 weeks (–1.38 s [p = 0.013]), 8 weeks (–1.20 s [p = 0.031]), 10 weeks (–1.58 s [p = 0.003]), and 12 weeks (–1.61 s [p = 0.001]) in the NL group. The TUG test improved significantly after 12 weeks (–1.41 s [p = 0.010]) in the N group. TUG test time improved significantly after 10 weeks (–1.25 s [p = 0.032]) and 12 weeks (–1.80 s [p = 0.001]) in the L group.
-
•
FTSST improved significantly after 2 weeks (–0.91 s [p = 0.034]), 4 weeks (–1.50 s [p = 0.002]), 6 weeks (–1.70 s [p = 0.002]), 8 weeks (–1.76 s [p = 0.001]), 10 weeks (–1.56 s [p = 0.005]), and 12 weeks (–2.14 s [p = 0.001]) in the NL group.
-
•
FTSST improved significantly after 4 weeks (–1.26 s [p = 0.026]), 6 weeks (–1.75 s [p = 0.005]), 8 weeks (–2.10 s [p = 0.001]), 10 weeks (–2.20 s [p = 0.001]), and 12 weeks (–2.31 s [p = 0.002]) in the N group.
-
•
FTSST improved significantly after 6 weeks (–1.42 s [p = 0.019]), 8 weeks (–1.36 s [p = 0.018]), 10 weeks (–1.82 s [p = 0.003]), and 12 weeks (–2.19 s [p = 0.001]) in the L group.
-
(2)
Comparison between the 3 groups (group difference in change from baseline) (Fig. 2).
-
•
Leg pain VAS score was improved significantly in the L group compared to the NL group after 6 weeks (16.2 mm [p = 0.029]) and 12 weeks (14.4 mm [p = 0.042]).
-
•
LBP VAS score was improved significantly in the N group compared to the NL group after 6 weeks (10.9 mm [p = 0.050]).
-
•
Walking speed was significantly greater in the NL group than in the N group after 2 weeks (0.25 m/s [p = 0.006]) and was significantly greater in the NL group than in the L group after 6 weeks (0.19 m/s [p = 0.045]).
-
•
RDQ scores were significantly lower in the L group than in the NL group after 8 weeks (2.1 [p = 0.024]).
-
(3)
Adverse events.
Adverse events occurred in 2 of 20 patients (10.0%) in group N: eczema in 2 patients (10.0%) and peripheral edema in 1 patient (5.0%). There were no adverse events for which a causal relationship to the study drug could not be excluded in the NL group and L group.
Comparison between the three groups (group difference in change from baseline). A Leg pain VAS score was significantly improved in the L group compared to the NL group after 6 weeks (16.2 mm [p = 0.029]) and 12 weeks (14.4 mm [p = 0.042]). B LBP VAS score was significantly improved in the N group compared to the NL group after 6 weeks (10.9 mm [p = 0.050]). C Walking speed was significantly greater in the NL group than in the N group after 2 weeks (0.25 m/s [p = 0.006]), and significantly greater in the NL group than in the L group after 6 weeks (0.19 m/s [p = 0.045]). D RDQ scores were significantly lower in the L group than in the NL group after 8 weeks (2.1 [p = 0.024])
Discussion
We investigated the effects of Neurotropin, limaprost, and combination therapy on clinical symptoms, QOL-related scores, psychological factors, walking activity, and standing balance in patients with LSS and low back pain.
Neurotropin improved LBP VAS score and RDQ, walking activity (walking speed and stride length), and standing balance (TUG and FTSST) and may contribute to the improvement of low back pain, walking activity, and standing balance.
By contrast, limaprost improved leg pain VAS values, LBP/QOL-related scores (ODI, EQ-5D-5L, and RDQ), psychological factors (PCS), and standing balance (TUG and FTSST), suggesting that limaprost may contribute to the improvement of leg pain, QOL-related scores, and standing balance.
Furthermore, the combined use of Neurotropin and limaprost improved walking activity (walking speed, stride length), standing balance (TUG and FTSST), LBP/QOL-related scores (ODI and RDQ), and psychological factors (PCS). In addition, the walking speed was improved compared with the N and L alone groups, suggesting an additional effect of the Neurotropin combination on walking ability.
Unlike nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, Neurotropin does not act on the prostaglandin production system or opioid system and has an excellent analgesic effect in rat chronic constriction injury (CCI) [12,13,14] and mouse spinal nerve ligation (SNL) [15], which are models of neuropathic pain.
The mechanism of analgesic action of Neurotropin is thought to be activation of the central analgesic mechanism of the descending pain-suppressive nerve system [10, 11], suppression of the release of the pain-producing substance bradykinin at the local noxious stimulus [16], and improvement of peripheral circulation.
Neurotropin effectively improved low back pain, walking activity, and standing balance in patients with LSS, and adverse events were as low as 10%. Because Neurotropin does not inhibit prostaglandin biosynthesis, side effects such as gastrointestinal disorders are few, and long-term administration to LSS patients is possible, which is expected to alleviate symptoms.
A randomized study showed that compared with NSAIDs, limaprost significantly improved leg numbness and walking distance characteristic of cauda equina LSS, but did not reduce LBP or leg pain [7]. By contrast, a randomized study of the effect of limaprost and NSAIDs in radicular-type LSS showed that the effect of limaprost was limited to radicular pain and had no effect on low back pain [8].
From the present study, Neurotropin was effective mainly in improving LBP, and limaprost was effective in leg pain. For LSS patients with LBP, the combined use of Neurotropin and limaprost could be considered to have a complementary effect on LBP and leg pain, and an additional effect of improving walking speed.
To date, there have been reports of improvement in subjective symptoms, but there have been no reports on the amount of walking-related activity. In the present study, we measured the amount of walking activity (walking speed and stride length) and standing balance (TUG and FTSST) using a measurement application that utilizes an iPhone’s GPS function. The combination of Neurotropin and limaprost improved walking speed compared with each drug alone. Furthermore, Neurotropin improved low back pain VAS value and RDQ, walking activity, and standing balance. By applying the GPS function of a smartphone app to determine the therapeutic effect of drugs, it may be possible to not only subjectively evaluate pain, but also objectively evaluate walking and balance functions.
Sarcopenia is a syndrome characterized by age-related loss of muscle mass, plus low muscle strength, and/or low physical performance. The Asian Working Group for Sarcopenia (AWGS) 2019 retains the previous definition of sarcopenia, but revises the diagnostic algorithm, protocols, and some criteria: low muscle strength is defined as handgrip strength < 28 kg for men and < 18 kg for women; criteria for low physical performance are 6-m walk < 1.0 m/s, or FTSST result ≥ 12 s [17].
Neurotropin improved reductions in physical performance, such as walking speed, standing balance, and TUG, which are diagnostic criteria for sarcopenia. Eguchi et al. reported that 46.6% of patients with degenerative lumbar scoliosis and 16% of patients with LSS had sarcopenia in older women with lumbar degenerative disease [18]. Neurotropin may lead to the treatment of sarcopenia in LSS patients.
This research has several imitations. First, there was no placebo group, so a placebo effect cannot be excluded. Second, the treatment period is short at 12 weeks, and it is necessary to verify the effects of long-term administration. Third, the sample size may be statistically too small to detect true direct differences between groups.
Conclusions
Combined use of Neurotropin and limaprost showed an additional effect on walking speed compared to single drug administration.
References
Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Jt Surg Br. 1954;36(2):230–7.
Arnoldi CC, Brodsky AE, Cauchoix J, et al. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res. 1976;115:4–5.
Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med. 2008;358(8):818–25.
Katz JN, Zimmerman ZE, Mass H, Makhni MC. Diagnosis and management of lumbar spinal stenosis: a review. JAMA. 2022;327(17):1688–99.
Ishimoto Y, Yoshimura N, Muraki S, et al. Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: the Wakayama Spine Study. Osteoarthr Cartil. 2012;20:1103–8.
Kobayashi S. Pathophysiology, diagnosis and treatment of intermittent claudication in patients with lumbar canal stenosis. World J Orthop. 2014;5:135–45.
Matsudaira K, Seichi A, Kunogi J, et al. The efficacy of prostaglandin E1 derivative in patients with lumbar spinal stenosis. Spine (Phila Pa 1976). 2009;34(2):115–20.
Onda A, Kikuchi S, Yabuki S, et al. Limaprost alfadex and nonsteroidal anti-inflammatory drugs for sciatica due to lumbar spinal stenosis. Eur Spine J. 2013;22(4):794–801.
Kim HJ, Kim JH, Park YS, et al. Comparative study of the efficacy of limaprost and pregabalin as single agents and in combination for the treatment of lumbar spinal stenosis: a prospective, double-blind, randomized controlled non-inferiority trial. Spine J. 2016;16(6):756–63.
Hata T, Kita T, Itoh E, Oyama R, Kawabata A. Mechanism of the analgesic effect of Neurotropin. Jpn J Pharmacol. 1988;48:165–73.
Kawamura M, Ohara H, Go K, Koga Y, Ienaga K. Neurotropin induces antinociceptive effect by enhancing descending pain inhibitory systems involving 5-HT3 and noradrenergic α2 receptors in spinal dorsal horn. Life Sci. 1998;62:2181–90.
Nishimoto S, Okada K, Tanaka H, et al. Neurotropin attenuates local inflammatory response and inhibits demyelination induced by chronic constriction injury of the mouse sciatic nerve. Biologicals. 2016;44:206–11.
Toda K, Muneshige H, Ikuta Y. Antinociceptive effects of Neurotropin in a rat model of painful peripheral mononeuropathy. Life Sci. 1998;62:913–21.
Saleh M, Muneshige H, Ikuta Y. Effects of Neurotropin on hyperalgesia and allodynia in mononeuropathic rats. Life Sci. 1998;63:1931–8.
Suzuki T, Li YH, Mashimo T. The antiallodynic and antihyperalgesic effects of Neurotropin in mice with spinal nerve ligation. Anesth Analg. 2005;101(3):793–9.
Ohara H, Namimatsu A, Fukuhara K, et al. Release of inflammatory mediators by noxious stimuli; effect of Neurotropin on the release. Eur J Pharmacol. 1988;157:93–9.
Chen LK, Woo J, Assantachai P, et al. Asian Working Group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300-307.e2.
Eguchi Y, Suzuki M, Yamanaka H, et al. Associations between sarcopenia and degenerative lumbar scoliosis in older women. Scoliosis Spinal Disord. 2017;12:9.
Acknowledgements
The authors wish to thank the participants of this study.
Funding
This study was supported by Nippon Zoki Pharmaceutical Co., Ltd., Osaka, Japan. Nippon Zoki Pharmaceutical Co., Ltd. was involved in the study design, planning of the data analysis, data interpretation, and development of the manuscript, but was not involved in data management and statistical analysis. EPS Co., Ltd. was directly involved in data management and statistical analysis. The study results were not influenced by the commercial sponsor of this study (Nippon Zoki Pharmaceutical Co., Ltd.).
Editorial Assistance
The authors would like to thank JAM post (https://www.jamp.com) for the English language review.
Author Contributions
Eguchi Y conducted data collection and data entry, and wrote the manuscript. Nakatani T (EPS Co., Ltd.) performed the statistical analysis. Eguchi Y, Aoki Y, Yamashita M, Fujimoto K, Sato T, Abe K, Sato M, Yamanaka H, Toyoguchi T, Shimizu K, Orita S, Inage K, Shiga Y, and Ohtori S collected patient data. All authors contributed to and approved the final manuscript.
Disclosures
Eguchi Y, Aoki Y, Yamashita M, Fujimoto K, Sato T, Abe K, Sato M, Yamanaka H, Toyoguchi T, Shimizu K, Orita S, Inage K, Shiga Y, and Ohtori S have nothing to disclose.
Compliance with Ethics Guidelines
The trial was conducted in accordance with the ethical principles, clinical research laws, and relevant notifications stipulated in the Declaration of Helsinki (as revised in 2013). The study received approval from the Clinical Research Review Committee, Chiba University Certified Review Board (CRB No. CRB0043-20), which notified all participating centers of the approval. This study was registered at the Japan Registry of Clinical Trials under the identifier jRCTs031200282. We declare that all participants provided written informed consent before their inclusion in this study.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Eguchi, Y., Aoki, Y., Yamashita, M. et al. Clinical Efficacy of Neurotropin for Lumbar Spinal Stenosis with Low Back Pain. Pain Ther 12, 461–473 (2023). https://doi.org/10.1007/s40122-022-00472-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00472-z