Abstract
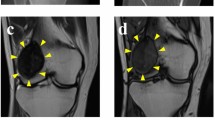
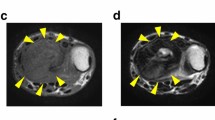
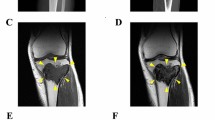
Giant cell tumor of bone (GCTB) is a rare osteolytic bone tumor consisting of mononuclear stromal cells, macrophages, and osteoclast-like giant cells. Although GCTB predominantly exhibits benign behavior, the tumor carries a significant risk of high local recurrence. Furthermore, GCTB can occasionally undergo malignant transformation and distal metastasis, making it potentially fatal. The standard treatment is complete surgical resection; nonetheless, an optimal treatment strategy for advanced GCTB remains unestablished, necessitating expanded preclinical research to identify appropriate therapeutic options. However, only one GCTB cell line is publicly available from a cell bank for research use worldwide. The present study reports the establishment of two novel cell lines, NCC-GCTB8-C1 and NCC-GCTB9-C1, derived from the primary tumor tissues of two patients with GCTB. Both cell lines maintained the hallmark mutation in the H3-3A gene, which is associated with tumor formation and development in GCTB. Characterization of these cell lines revealed their steady growth, spheroid-formation capability, and invasive traits. Potential therapeutic agents were identified via extensive drug screening of the two cell lines and seven previously established GCTB cell lines. Among the 214 antitumor agents tested, romidepsin, a histone deacetylase inhibitor, and mitoxantrone, a topoisomerase inhibitor, were identified as potential therapeutic agents against GCTB. Conclusively, the establishment of NCC-GCTB8-C1 and NCC-GCTB9-C1 provides novel and crucial resources that are expected to advance GCTB research and potentially revolutionize treatment strategies.






Similar content being viewed by others
Data availability
Data will be available under reasonable conditions.
References
board WHOcte. Soft tissue and bone tumours. 5th ed. World Health Organization classification of tumours. World Health Organization. International Agency for Research on Cancer; 2020.
Nagano A, Urakawa H, Tanaka K, Ozaki T. Current management of giant-cell tumor of bone in the denosumab era. Jpn J Clin Oncol. 2022;52(5):411–6. https://doi.org/10.1093/jjco/hyac018.
Turcotte RE. Giant cell tumor of bone. Orthop Clin North Am. 2006;37(1):35–51. https://doi.org/10.1016/j.ocl.2005.08.005.
Rekhi B, Dave V. Giant cell tumor of bone: an update, including spectrum of pathological features, pathogenesis, molecular profile and the differential diagnoses. Histol Histopathol. 2023;38(2):139–53. https://doi.org/10.14670/hh-18-486.
Basu Mallick A, Chawla SP. Giant cell tumor of bone: an update. Curr Oncol Rep. 2021;23(5):51. https://doi.org/10.1007/s11912-021-01047-5.
Noguchi R, Yoshimatsu Y, Ono T, Sei A, Hirabayashi K, Ozawa I, et al. Establishment and characterization of NCC-GCTB1-C1: a novel patient-derived cancer cell line of giant cell tumor of bone. Hum Cell. 2020;33(4):1321–8. https://doi.org/10.1007/s13577-020-00415-w.
Yamamoto H, Ishihara S, Toda Y, Oda Y. Histone H3.3 mutation in giant cell tumor of bone: an update in pathology. Med Mol Morphol. 2020;53(1):1–6. https://doi.org/10.1007/s00795-019-00238-1.
Taylor EL, Westendorf JJ. Histone mutations and bone cancers. Adv Exp Med Biol. 2021;1283:53–62. https://doi.org/10.1007/978-981-15-8104-5_4.
Yang L, Zhang H, Zhang X, Tang Y, Wu Z, Wang Y, et al. Clinicopathologic and molecular features of denosumab-treated giant cell tumour of bone (GCTB): analysis of 21 cases. Ann Diagn Pathol. 2022;57: 151882. https://doi.org/10.1016/j.anndiagpath.2021.151882.
Li H, Gao J, Gao Y, Lin N, Zheng M, Ye Z. Denosumab in giant cell tumor of bone: current status and pitfalls. Front Oncol. 2020;10: 580605. https://doi.org/10.3389/fonc.2020.580605.
Lin X, Liu J, Xu M. The prognosis of giant cell tumor of bone and the vital risk factors that affect its postoperative recurrence: a meta-analysis. Transl Cancer Res. 2021;10(4):1712–22. https://doi.org/10.21037/tcr-20-3100.
Yang Y, Huang Z, Niu X, Xu H, Li Y, Liu W. Clinical characteristics and risk factors analysis of lung metastasis of benign giant cell tumor of bone. J Bone Oncol. 2017;7:23–8. https://doi.org/10.1016/j.jbo.2017.04.001.
Palmerini E, Picci P, Reichardt P, Downey G. Malignancy in giant cell tumor of bone: a review of the literature. Technol Cancer Res Treat. 2019;18:1533033819840000. https://doi.org/10.1177/1533033819840000.
Muheremu A, Niu X. Pulmonary metastasis of giant cell tumor of bones. World J Surg Oncol. 2014;12:261. https://doi.org/10.1186/1477-7819-12-261.
Aponte-Tinao LA, Piuzzi NS, Roitman P, Farfalli GL. A High-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin Orthop Relat Res. 2015;473(9):3050–5. https://doi.org/10.1007/s11999-015-4249-2.
Kondo T. Current status and future outlook for patient-derived cancer models from a rare cancer research perspective. Cancer Sci. 2021;112(3):953–61. https://doi.org/10.1111/cas.14669.
Hattori E, Oyama R, Kondo T. Systematic review of the current status of human sarcoma cell lines. Cells. 2019;8(2):157. https://doi.org/10.3390/cells8020157.
Goodspeed A, Heiser LM, Gray JW, Costello JC. Tumor-derived cell lines as molecular models of cancer pharmacogenomics. Mol Cancer Res. 2016;14(1):3–13. https://doi.org/10.1158/1541-7786.Mcr-15-0189.
Bairoch A. The cellosaurus, a cell-line knowledge resource. J Biomol Tech. 2018;29(2):25–38. https://doi.org/10.7171/jbt.18-2902-002.
Drexler HG, Dirks WG, MacLeod RA, Uphoff CC. False and mycoplasma-contaminated leukemia-lymphoma cell lines: time for a reappraisal. Int J Cancer. 2017;140(5):1209–14. https://doi.org/10.1002/ijc.30530.
Ono T, Noguchi R, Yoshimatsu Y, Tsuchiya R, Sin Y, Nakagawa R, et al. Establishment and characterization of the NCC-GCTB4-C1 cell line: a novel patient-derived cell line from giant cell tumor of bone. Hum Cell. 2022;35(1):392–9. https://doi.org/10.1007/s13577-021-00639-4.
Ono T, Noguchi R, Yoshimatsu Y, Sin Y, Tsuchiya R, Akiyama T, et al. Establishment and characterization of two novel patient-derived cell lines from giant cell tumor of bone. Hum Cell. 2023;36(5):1804–12. https://doi.org/10.1007/s13577-023-00928-0.
Yoshimatsu Y, Noguchi R, Tsuchiya R, Ono T, Sin Y, Akane S, et al. Establishment and characterization of novel patient-derived cell lines from giant cell tumor of bone. Hum Cell. 2021;34(6):1899–910. https://doi.org/10.1007/s13577-021-00579-z.
Akiyama T, Yoshimatsu Y, Noguchi R, Sin Y, Tsuchiya R, Ono T, et al. Establishment and characterization of NCC-GCTB5-C1: a novel cell line of giant cell tumor of bone. Hum Cell. 2022;35(5):1621–9. https://doi.org/10.1007/s13577-022-00724-2.
Gonçalves E, Poulos RC, Cai Z, Barthorpe S, Manda SS, Lucas N, et al. Pan-cancer proteomic map of 949 human cell lines. Cancer Cell. 2022;40(8):835-49.e8. https://doi.org/10.1016/j.ccell.2022.06.010.
Kinker GS, Greenwald AC, Tal R, Orlova Z, Cuoco MS, McFarland JM, et al. Pan-cancer single-cell RNA-seq identifies recurring programs of cellular heterogeneity. Nat Genet. 2020;52(11):1208–18. https://doi.org/10.1038/s41588-020-00726-6.
Jiang G, Zhang S, Yazdanparast A, Li M, Pawar AV, Liu Y, et al. Comprehensive comparison of molecular portraits between cell lines and tumors in breast cancer. BMC Genomics. 2016;17(Suppl 7):525. https://doi.org/10.1186/s12864-016-2911-z.
Karpik M. Giant cell tumor (tumor gigantocellularis, osteoclastoma)—epidemiology, diagnosis, treatment. Ortop Traumatol Rehabil. 2010;12(3):207–15.
Lim J, Park JH, Baude A, Yoo Y, Lee YK, Schmidt CR, et al. The histone variant H3.3 G34W substitution in giant cell tumor of the bone link chromatin and RNA processing. Sci Rep. 2017;7(1):13459. https://doi.org/10.1038/s41598-017-13887-y.
Wang Y, Jeon H. 3D cell cultures toward quantitative high-throughput drug screening. Trends Pharmacol Sci. 2022;43(7):569–81. https://doi.org/10.1016/j.tips.2022.03.014.
Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4(3):309–24. https://doi.org/10.1038/nprot.2008.226.
Shimony S, Horowitz N, Ribakovsky E, Rozovski U, Avigdor A, Zloto K, et al. Romidepsin treatment for relapsed or refractory peripheral and cutaneous T-cell lymphoma: real-life data from a national multicenter observational study. Hematol Oncol. 2019;37(5):569–77. https://doi.org/10.1002/hon.2691.
Venneker S, van Eenige R, Kruisselbrink AB, Palubeckaitė I, Taliento AE, Briaire-de Bruijn IH, et al. Histone deacetylase inhibitors as a therapeutic strategy to eliminate neoplastic “stromal” cells from giant cell tumors of bone. Cancers (Basel). 2022;14(19):4708. https://doi.org/10.3390/cancers14194708.
Yafei J, Haoran M, Wenyan J, Linghang X, Kai T, Gangyang W, et al. Personalized medicine modality based on patient-derived xenografts from a malignant transformed GCTB harboring H3F3A G34W mutation. J Orthop Translat. 2021;29:106–12. https://doi.org/10.1016/j.jot.2021.04.004.
Evison BJ, Sleebs BE, Watson KG, Phillips DR, Cutts SM. Mitoxantrone, more than just another topoisomerase II poison. Med Res Rev. 2016;36(2):248–99. https://doi.org/10.1002/med.21364.
Acknowledgements
We thank Drs. S. Fukushima, S. Osaki, S. Iwata (Department of Musculoskeletal Oncology and Rehabilitation), and Y. Takahashi (Department of Diagnostic Pathology), and the National Cancer Center Hospital for sampling the tumor tissue specimens from surgically resected materials. We appreciate the technical assistance provided by Mr. Y. Ohno (Division of Rare Cancer Research, National Cancer Center Research Institute) and Mrs. Y. Shiotani (Central Animal Division, National Cancer Center Research Institute). We would like to thank Editage (www.editage.jp) for providing English language editing services and for their constructive comments on the manuscript. Technical assistance for this study was provided by the Fundamental Innovative Oncology Core of the National Cancer Center.
Funding
This study was supported by the Japan Agency for Medical Research and Development (Grant Number: 20ck0106537h).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The use of clinical materials for this study was approved by the ethics committee of the National Cancer Center (approval number: 2004-050).
Informed consent
Written informed consent was provided by the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adachi, Y., Noguchi, R., Yoshimatsu, Y. et al. Establishment and characterization of two novel patient-derived cell lines from giant cell tumor of bone: NCC-GCTB8-C1 and NCC-GCTB9-C1. Human Cell 37, 874–885 (2024). https://doi.org/10.1007/s13577-024-01042-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-024-01042-5