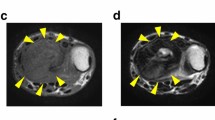
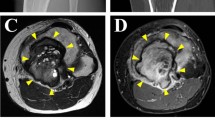
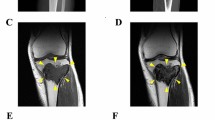
Abstract
Giant cell tumor of bone (GCTB), is a rare intermediate malignant bone tumor with high local infiltrative ability, and is genetically characterized by mutation in the H3-3A gene. Standard treatment is curative surgical tumor resection. GCTB demonstrates both local recurrence and pulmonary metastasis after surgical treatment, and effective systematic chemotherapy is yet to be established. Therefore, development of novel chemotherapies for GCTB is necessary. Although patient-derived tumor cell lines are potent tools for preclinical research, 15 GCTB cell lines have been reported to date, and only four are publicly available. Thus, this study aimed to establish and characterize a novel GCTB cell line for preclinical studies on GCTB. Herein, we described the establishment of a cell line, NCC-GCTB5-C1, from the primary tumor tissue of a patient with GCTB. NCC-GCTB5-C1 was shown to harbor a mutation in the H3-3A gene, which is typical of GCTB; thus, it has useful properties for in vitro studies. We conducted the largest integrated screening analysis of 214 antitumor agents using NCC-GCTB5-C1 along with four GCTB cell lines. Romidepsin (a histone deacetylase inhibitor), camptothecin, and actinomycin D (topoisomerase inhibitors) demonstrated remarkable antitumor effects, suggesting that these antitumor agents are potential therapeutic candidates for GCTB treatment. Therefore, the NCC-GCTB5-C1 cell line could potentially contribute to the elucidation of GCTB pathogenesis and the development of novel GCTB treatments.





Similar content being viewed by others
Change history
18 October 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13577-022-00805-2
References
van der Heijden L, Dijkstra PD, van de Sande MA, et al. The clinical approach toward giant cell tumor of bone. Oncologist. 2014;19:550–61.
López-Pousa A, Martín Broto J, Garrido T, Vázquez J. Giant cell tumour of bone: new treatments in development. Clin Transl Oncol. 2015;17:419–30.
Tsukamoto S, Mavrogenis AF, Kido A, Errani C. Current concepts in the treatment of giant cell tumors of bone. Cancers (Basel). 2021;13:3647.
Basu Mallick A, Chawla SP. Giant cell tumor of bone: an update. Curr Oncol Rep. 2021;23:51.
Liede A, Bach BA, Stryker S, et al. Regional variation and challenges in estimating the incidence of giant cell tumor of bone. J Bone Jt Surg Am. 2014;96:1999–2007.
Cleven AH, Höcker S, Briaire-de Bruijn I, Szuhai K, Cleton-Jansen AM, Bovée JV. Mutation analysis of H3F3A and H3F3B as a diagnostic tool for giant cell tumor of bone and chondroblastoma. Am J Surg Pathol. 2015;39:1576–83.
Presneau N, Baumhoer D, Behjati S, et al. Diagnostic value of H3F3A mutations in giant cell tumour of bone compared to osteoclast-rich mimics. J Pathol Clin Res. 2015;1:113–23.
Amary F, Berisha F, Ye H, et al. H3F3A (Histone 3.3) G34W immunohistochemistry: a reliable marker defining benign and malignant giant cell tumor of bone. Am J Surg Pathol. 2017;41:1059–68.
Behjati S, Tarpey PS, Presneau N, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet. 2013;45:1479–82.
Chakarun CJ, Forrester DM, Gottsegen CJ, Patel DB, White EA, Matcuk GR Jr. Giant cell tumor of bone: review, mimics, and new developments in treatment. Radiographics. 2013;33:197–211.
Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36:1–7.
Jamshidi K, Gharehdaghi M, Hajialiloo SS, Mirkazemi M, Ghaffarzadehgan K, Izanloo A. Denosumab in patients with giant cell tumor and its recurrence: a systematic review. Arch Bone Jt Surg. 2018;6:260–8.
Chan CM, Adler Z, Reith JD, Gibbs CP Jr. Risk factors for pulmonary metastases from giant cell tumor of bone. J Bone Jt Surg Am. 2015;97:420–8.
Dominkus M, Ruggieri P, Bertoni F, et al. Histologically verified lung metastases in benign giant cell tumours–14 cases from a single institution. Int Orthop. 2006;30:499–504.
Wang J, Liu X, Yang Y, et al. Pulmonary metastasis of giant cell tumour: a retrospective study of three hundred and ten cases. Int Orthop. 2021;45:769–78.
Goodspeed A, Heiser LM, Gray JW, Costello JC. Tumor-derived cell lines as molecular models of cancer pharmacogenomics. Mol Cancer Res. 2016;14:3–13.
Tseng YY, Boehm JS. From cell lines to living biosensors: new opportunities to prioritize cancer dependencies using ex vivo tumor cultures. Curr Opin Genet Dev. 2019;54:33–40.
Hattori E, Oyama R, Kondo T. Systematic review of the current status of human sarcoma cell lines. Cells. 2019;8:157.
Hideyuki T, Yoko T, Daisuke M, Miharu K, Tohru M, Hiroshi M. Cell line individualization by STR multiplex system in the cell bank found cross-contamination between ECV304 and EJ-1/T24. Tissue Cult Res Commun. 1999;18:329–38.
Masters JR, Thomson JA, Daly-Burns B, et al. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci U S A. 2001;98:8012–7.
Drexler HG, Dirks WG, MacLeod RA, Uphoff CC. False and mycoplasma-contaminated leukemia-lymphoma cell lines: time for a reappraisal. Int J Cancer. 2017;140:1209–14.
Billiau A, Edy VG, Heremans H, et al. Human interferon: mass production in a newly established cell line, MG-63. Antimicrob Agents Chemother. 1977;12:11–5.
Cox C, Teknos TN, Barrios M, Brewer GJ, Dick RD, Merajver SD. The role of copper suppression as an antiangiogenic strategy in head and neck squamous cell carcinoma. Laryngoscope. 2001;111:696–701.
Noguchi R, Yoshimatsu Y, Ono T, et al. Establishment and characterization of NCC-GCTB1-C1: a novel patient-derived cancer cell line of giant cell tumor of bone. Hum Cell. 2020;33:1321–8.
Yoshimatsu Y, Noguchi R, Tsuchiya R, et al. Establishment and characterization of novel patient-derived cell lines from giant cell tumor of bone. Hum Cell. 2021;34:1899–910.
Ono T, Noguchi R, Yoshimatsu Y, et al. Establishment and characterization of the NCC-GCTB4-C1 cell line: a novel patient-derived cell line from giant cell tumor of bone. Hum Cell. 2022;35:392–9.
Suraweera A, O’Byrne KJ, Richard DJ. Combination therapy with histone deacetylase inhibitors (HDACi) for the treatment of cancer: achieving the full therapeutic potential of HDACi. Front Oncol. 2018;8:92.
Skok Ž, Zidar N, Kikelj D, Ilaš J. Dual inhibitors of human DNA topoisomerase II and other cancer-related targets. J Med Chem. 2020;63:884–904.
Zarin DA, Fain KM, Dobbins HD, Tse T, Williams RJ. 10-year update on study results submitted to ClinicalTrials.gov. N Engl J Med. 2019;381:1966–74.
Shinn P, Chen L, Ferrer M, et al. High-throughput screening for drug combinations. Methods Mol Biol. 2019;1939:11–35.
Marchion DC, Bicaku E, Turner JG, Daud AI, Sullivan DM, Munster PN. Synergistic interaction between histone deacetylase and topoisomerase II inhibitors is mediated through topoisomerase IIbeta. Clin Cancer Res. 2005;11:8467–75.
Marchion DC, Bicaku E, Daud AI, Richon V, Sullivan DM, Munster PN. Sequence-specific potentiation of topoisomerase II inhibitors by the histone deacetylase inhibitor suberoylanilide hydroxamic acid. J Cell Biochem. 2004;92:223–37.
Acknowledgements
We thank Drs. E. Kobayashi, S. Iwata, K.Ogura, S.Osaki, K.Sato, C.Sato, S.Ishihara (Department of Musculoskeletal Oncology), and the National Cancer Center Hospital for sampling tumor tissue specimens from surgically resected materials. We also appreciate the technical assistance provided by Mrs. Y. Kuwata (Division of Rare Cancer Research) and technical support provided by Mrs. Y. Shiotani, Mr. N. Uchiya, and Dr. T. Imai (Central Animal Division, National Cancer Center Research Institute). We would like to thank Editage (www.editage.jp) for providing English language editing services and for their constructive comments on this manuscript. This study was technically assisted by the Fundamental Innovative Oncology Core of the National Cancer Center.
Funding
This research was supported by the Japan Agency for Medical Research and Development (Grant number: 20ck0106537h0002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The ethical committee of the National Cancer Center approved the use of clinical materials for this study (approval number: 2004-050). Animal experiments were conducted in compliance with the guidelines of the Institute for Laboratory Animal Research, National Cancer Center Research Institute.
Informed consent
Written informed consent was provided by the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13577_2022_724_MOESM1_ESM.tiff
Supplementary file1 Short tandem repeat patterns of NCC-GCTB5-C1 and the original tumor tissue (a) Short tandem repeat patterns of the original tumor tissue of NCC-GCTB5-C1 and (b) short tandem repeat patterns of NCC-GCTB5-C1 cells (passage 25) (TIFF 3925 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akiyama, T., Yoshimatsu, Y., Noguchi, R. et al. Establishment and characterization of NCC-GCTB5-C1: a novel cell line of giant cell tumor of bone. Human Cell 35, 1621–1629 (2022). https://doi.org/10.1007/s13577-022-00724-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00724-2