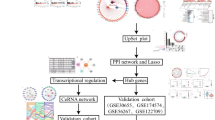
Abstract
Intracranial aneurysm (IA) has the potential to rupture. Despite scientific advances, we are still not in a position to screen patients for IA and identify those at risk of rupture. It is critical to comprehend the molecular basis of disease to facilitate the development of novel diagnostic strategies. We used transcriptomics to identify the dysregulated genes and understand their role in the disease biology. In particular, RNA-Seq was performed in tissue samples of controls, unruptured IA, and ruptured IA. Dysregulated genes (DGs) were identified and analyzed to understand the functional aspects of molecules. Subsequently, candidate genes were validated at both transcript and protein level. There were 314 DGs in patients with unruptured IA when compared to control samples. Out of these, SPARC and OSM were validated as candidate molecules in unruptured IA. PI3K-AKT signaling pathway was found to be an important pathway for the formation of IA. Similarly, 301 DGs were identified in the samples of ruptured IA when compared with unruptured IAs. CTSL was found to be a key candidate molecule which along with Hippo signaling pathway may be involved in the rupture of IA. We conclude that activation of PI3K-AKT signaling pathway by OSM along with up-regulation of SPARC is important for the formation of IA. Further, regulation of Hippo pathway through PI3K-AKT signaling results in the down-regulation of YAP1 gene. This along with up-regulation of CTSL leads to further weakening of aneurysm wall and its subsequent rupture.







Similar content being viewed by others
Data Availability
The datasets generated for this study can be found in the Sequence Read Archive hosted by National Center for Biotechnology Information Search database (NCBI) with accession number: PRJNA524023.
References
Thompson JW, Elwardany O, McCarthy DJ, Sheinberg DL, Alvarez CM, Nada A, et al. In vivo cerebral aneurysm models. Neurosurg Focus. 2019;47:E20. https://doi.org/10.3171/2019.4.FOCUS19219.
Wu Y, Li Z, Shi Y, Chen L, Tan H, Wang Z, et al. Exome sequencing identifies LOXL2 mutation as a cause of familial intracranial aneurysm. World Neurosurg. 2018;109:e812–8. https://doi.org/10.1016/j.wneu.2017.10.094.
Song Y, Lee JK, Lee JO, Kwon B, Seo EJ, Suh DC. Whole exome sequencing in patients with phenotypically associated familial intracranial aneurysm. Korean J Radiol. 2022;23:101–11. https://doi.org/10.3348/kjr.2021.0467.
Tromp G, Weinsheimer S, Ronkainen A, Kuivaniemi H. Molecular basis and genetic predisposition to intracranial aneurysm. Ann Med. 2014;46:597–606. https://doi.org/10.3109/07853890.2014.949299.
Sharma T, Datta KK, Kumar M, Dey G, Khan AA, Mangalaparthi KK, et al. Intracranial aneurysm biomarker candidates identified by a proteome-wide study. OMICS. 2020;24:483–92. https://doi.org/10.1089/omi.2020.0057.
Wang J, Yu L, Huang X, Wang Y, Zhao J. Comparative proteome analysis of saccular intracranial aneurysms with iTRAQ quantitative proteomics. J Proteomics. 2016;130:120–8. https://doi.org/10.1016/j.jprot.2015.09.014.
Sakaya GR, Parada CA, Eichler RA, Yamaki VN, Navon A, Heimann AS, et al. Peptidomic profiling of cerebrospinal fluid from patients with intracranial saccular aneurysms. J Proteomics. 2021;240:104188. https://doi.org/10.1016/j.jprot.2021.104188.
Poppenberg KE, Li L, Waqas M, Paliwal N, Jiang K, Jarvis JN, et al. Whole blood transcriptome biomarkers of unruptured intracranial aneurysm. PLoS One. 2020;15:e0241838. https://doi.org/10.1371/journal.pone.0241838.
Tutino VM, Zebraski HR, Rajabzadeh-Oghaz H, Waqas M, Jarvis JN, Bach K, et al. Identification of circulating gene expression signatures of intracranial aneurysm in peripheral blood mononuclear cells. Diagnostics (Basel). 2021;11:1092. https://doi.org/10.3390/diagnostics11061092.
Fan J, Yu L, Zhao J. Comparative transcriptome analysis reveals involvement of TLR-2 signaling in the pathogenesis of intracranial aneurysm. J Clin Neurosci. 2018;47:258–63. https://doi.org/10.1016/j.jocn.2017.07.016.
Kleinloog R, Verweij BH, van der Vlies P, Deelen P, Swertz MA, de Muynck L, et al. RNA sequencing analysis of intracranial aneurysm walls reveals involvement of lysosomes and immunoglobulins in rupture. Stroke. 2016;47:1286–93. https://doi.org/10.1161/STROKEAHA.116.012541.
Kurki MI, Hakkinen SK, Frosen J, Tulamo R, von Und Zu Fraunberg M, Wong G, et al. Upregulated signaling pathways in ruptured human saccular intracranial aneurysm wall: an emerging regulative role of toll-like receptor signaling and nuclear factor-kB, hypoxia-inducible factor-1A, and ETS transcription factors. Neurosurgery. 2011;68:1667–75. https://doi.org/10.1227/NEU.0b013e318210f001.
Nakaoka H, Tajima A, Yoneyama T, Hosomichi K, Kasuya H, Mizutani T, et al. Gene expression profiling reveals distinct molecular signatures associated with the rupture of intracranial aneurysm. Stroke. 2014;45(8):2239–45. https://doi.org/10.1161/STROKEAHA.114.005851.
Pera J, Korostynski M, Krzyszkowski T, Czopek J, Slowik A, Dziedzic T, et al. Gene expression profiles in human ruptured and unruptured intracranial aneurysms. What Is the Role of Inflammation? Stroke. 2010;41:224–31. https://doi.org/10.1161/STROKEAHA.109.562009.
Jiang Y, Zhang M, He H, Chen J, Zeng H, Li J, et al. MicroRNA/mRNA profiling and regulatory network of intracranial aneurysm. BMC Med Genomics. 2013;6:36. https://doi.org/10.1186/1755-8794-6-36.
Tutino VM, Zebraski HR, Rajabzadeh-Oghaz H, Chaves L, Dmytriw AA, Siddiqui AH, et al. RNA sequencing data from human intracranial aneurysm tissue reveals a complex inflammatory environment associated with rupture. Mol Diagn Ther. 2021;25:775–90. https://doi.org/10.1007/s40291-021-00552-4.
Chen X, Yang S, Yang J, Liu Q, Li M, Wu J, et al. Circular RNA circDUS2 is a potential biomarker for intracranial aneurysm. Front Aging Neurosci. 2021;13:632448. https://doi.org/10.3389/fnagi.2021.63244.
Chen S, Li M, Xin W, Liu S, Zheng L, Li Y, et al. Intracranial aneurysm's association with genetic variants, transcription abnormality, and methylation changes in ADAMTS genes. PeerJ. 2020;8:e8596. https://doi.org/10.7717/peerj.8596.
Lorenzo-Betancor O, Blackburn PR, Edwards E, Vázquez-do-Campo R, Klee EW, Labbé C, et al. PCNT point mutations and familial intracranial aneurysms. Neurology. 2018;91:e217081. https://doi.org/10.1212/WNL.0000000000006614.
Aoki T, Koseki H, Miyata H, Itoh M, Kawaji H, Takizawa K, et al. RNA sequencing analysis revealed the induction of CCL3 expression in human intracranial aneurysms. Sci Rep. 2019;9:10387. https://doi.org/10.1038/s41598-019-46886-2.
Andrews S (2010). FastQC: a quality control tool for high throughput sequence data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc
Pertea G. 2015. Fqtrim: v0.9.4 release. https://ccb.jhu.edu/software/fqtrim/
Kim D, Langmead B, Salzberg SL. HISAT: A fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60. https://doi.org/10.1038/nmeth.3317.
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. Stringtie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33:290–5. https://doi.org/10.1038/nbt.3122.
Anders S, Pyl PT, Huber W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–9. https://doi.org/10.1093/bioinformatics/btu638.
Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. https://doi.org/10.1186/gb-2010-11-10-r106.
Pathan M, Keerthikumar S, Ang CS, Gangoda L, Quek CY, Williamson NA, et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics. 2015;15:2597–601. https://doi.org/10.1002/pmic.201400515.
Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. https://doi.org/10.1038/nprot.2008.211.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–13. https://doi.org/10.1093/nar/gky1131.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. https://doi.org/10.1101/gr.1239303.
Mi H, Muruganujan A, Huang X, Ebert D, Mills C, Guo X, et al. Protocol update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat Protoc. 2019;14:703–21. https://doi.org/10.1038/s41596-019-0128-8.
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-blast: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. https://doi.org/10.1186/1471-2105-13-134.
Bekelis K, Kerley-Hamilton JS, Teegarden A, Tomlinson CR, Kuintzle R, Simmons N, et al. MicroRNA and gene expression changes in unruptured human cerebral aneurysms. J Neurosurg. 2016;125:1390–9. https://doi.org/10.3171/2015.11.JNS151841.
Li H, Wang W, Zhang L, Lan Q, Wang J, Cao Y, et al. Identification of a long noncoding RNA-associated competing endogenous RNA network in intracranial aneurysm. World Neurosurg. 2017;97:684–692.e4. https://doi.org/10.1016/j.wneu.2016.10.016.
Li Z, Tan H, Shi Y, Huang G, Wang Z, Liu L, et al. Global gene expression patterns and somatic mutations in sporadic intracranial aneurysms. World Neurosurg. 2017;100:15–21. https://doi.org/10.1016/j.wneu.2016.12.109.
Laarman MD, Kleinloog R, Bakker MK, Rinkel GJ, Bakkers J, Ruigrok YM. Assessment of the most optimal control tissue for intracranial aneurysm gene expression studies. Stroke. 2019;50:2933–6. https://doi.org/10.1161/STROKEAHA.119.024881.
Aoki T, Frosen J, Fukuda M, Bando K, Shioi G, Tsuji K, et al. Prostaglandin E2-EP2-NF-κB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Sci Signal. 2017;10(465):eaah6037. https://doi.org/10.1126/scisignal.aah6037.
Laaksamo E, Tulamo R, Baumann M, Dashti R, Hernesniemi J, Juvela S, et al. Involvement of mitogen-activated protein kinase signaling in growth and rupture of human intracranial aneurysms. Stroke. 2008;39:886–92. https://doi.org/10.1161/STROKEAHA.107.497875.
Wang L, Chen Y, Sternberg P, Cai J. Essential roles of the PI3 Kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Invest Ophthalmol Vis Sci. 2008;49:1671–8. https://doi.org/10.1167/iovs.07-1099.
Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z, et al. PI3K/Akt pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020;11:797. https://doi.org/10.1038/s41419-020-02998-6.
Zhao Y, Qian Y, Sun Z, Shen X, Cai Y, Li L, et al. Role of PI3K in the progression and regression of atherosclerosis. Front Pharmacol. 2021;12:632378. https://doi.org/10.3389/fphar.2021.632378.
Penn DL, Witte SR, Komotar RJ, Connolly ES Jr. The role of vascular remodeling and inflammation in the pathogenesis of intracranial aneurysms. J Clin Neurosci. 2014;21:28–32. https://doi.org/10.1016/j.jocn.2013.07.004.
Greene MA, Loeser RF. Function of the chondrocyte PI-3 kinase-Akt signaling pathway is stimulus dependent. Osteoarthritis Cartilage. 2015;23:949–56. https://doi.org/10.1016/j.joca.2015.01.014.
Li MH, Li PG, Huang QL, Ling J. Endothelial injury preceding intracranial aneurysm formation in rabbits. West Indian Med J. 2014;63:167–71. https://doi.org/10.7727/wimj.2013.129.
Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013;44:3613–22. https://doi.org/10.1161/STROKEAHA.113.002390.
Turkmani AH, Edwards NJ, Chen PR. The role of inflammation in cerebral aneurysms. Neuroimmunol Neuroinflammation. 2015;2:102–6.
Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells-- conditional innate immune cells. J Hematol Oncol. 2013;6:61. https://doi.org/10.1186/1756-8722-6-61.
Rega G, Kaun C, Weiss TW, Demyanets S, Zorn G, Kastl SP, et al. Inflammatory cytokines interleukin-6 and oncostatin-M induce plasminogen activator inhibitor-1 in human adipose tissue. Circulation. 2005;111:1938–45. https://doi.org/10.1161/01.CIR.0000161823.55935.BE.
Setiadi H, Yago T, Liu Z, McEver RP. Endothelial signaling by neutrophil-released oncostatin M enhances P-selectin-dependent inflammation and thrombosis. Blood Adv. 2019;3:168–83. https://doi.org/10.1182/bloodadvances.2018026294.
Bou-Gharios G, Ponticos M, Rajkumar V, Abraham D. Extra-cellular matrix in vascular networks. Cell Prolif. 2004;37:207–20. https://doi.org/10.1111/j.1365-2184.2004.00306.x.
Phan E, Ahluwalia A, Tarnawski AS. Role of SPARC-matricellular protein in pathophysiology and tissue injury healing. Implications for gastritis and gastric ulcers. Med Sci Monit. 2007;13:RA25-30.
Alkabie S, Basivireddy J, Zhou L, Roskams J, Rieckmann P, Quandt JA. SPARC expression by cerebral microvascular endothelial cells in vitro and its influence on blood-brain barrier properties. J Neuroinflammation. 2016;13:225. https://doi.org/10.1186/s12974-016-0657-9.
Li B, Li F, Chi L, Zhang L, Zhu S. The expression of SPARC in human intracranial aneurysms and its relationship with MMP-2/-9. PLoS One. 2013;8:e58490. https://doi.org/10.1371/journal.pone.0058490.
McClung HM, Thomas SL, Osenkowski P, Toth M, Menon P, Raz A, et al. SPARC upregulates MT1-MMP expression, MMP-2 activation, and the secretion and cleavage of Galectin-3 in U87MG glioma cells. Neurosci Lett. 2007;419:172–7. https://doi.org/10.1016/j.neulet.2007.04.037.
Seet LF, Su R, Toh LZ, Wong TT. In vitro analyses of the anti-fibrotic effect of SPARC silencing in human Tenon’s fibroblasts: comparisons with mitomycin C. J Cell Mol Med. 2012;16:1245–59. https://doi.org/10.1111/j.1582-4934.2011.01400.x.
Zhou S, Dion PA, Rouleau GA. Genetics of intracranial aneurysms. Stroke. 2018;49:780–7. https://doi.org/10.1161/STROKEAHA.117.018152.
He J, Bao Q, Yan M, Liang J, Zhu Y, Wang C, et al. The role of Hippo/yes-associated protein signalling in vascular remodelling associated with cardiovascular disease. Br J Pharmacol. 2018;175:1354–61. https://doi.org/10.1016/j.joca.2015.01.014.
Jiang WJ, Ren WH, Liu XJ, Liu Y, Wu FJ, Sun LZ, et al. Disruption of mechanical stress in extracellular matrix is related to Stanford type A aortic dissection through down-regulation of Yes-associated protein. Aging (Albany NY). 2016;8:1923–39. https://doi.org/10.18632/aging.101033.
Ponticos M, Smith BD. Extracellular matrix synthesis in vascular disease: hypertension, and atherosclerosis. J Biomed Res. 2014;28(1):25. https://doi.org/10.7555/JBR.27.20130064.
Miyata T, Minami M, Kataoka H, Hayashi K, Ikedo T, Yang T, et al. Osteoprotegerin prevents intracranial aneurysm progression by promoting collagen biosynthesis and vascular smooth muscle cell proliferation. J Am Heart Assoc. 2020;9:e015731. https://doi.org/10.1161/JAHA.119.015731.
Sun J, Sukhova GK, Zhang J, Chen H, Sjoberg S, Libby P, et al. Cathepsin L activity is essential to elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2011;31:2500–8. https://doi.org/10.1161/ATVBAHA.111.230201.
Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, et al. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115:2065–75. https://doi.org/10.1161/CIRCULATIONAHA.107.688523.
Liu J, Sukhova GK, Yang JT, Sun J, Ma L, Ren A, et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006;184:302–11. https://doi.org/10.1016/j.atherosclerosis.2005.05.012.
Takeda T, Yamamoto Y, Tsubaki M, Matsuda T, Kimura A, Shimo N, et al. PI3K/Akt/YAP signaling promotes migration and invasion of DLD-1 colorectal cancer cells. Oncol Lett. 2022;23:106. https://doi.org/10.3892/ol.2022.13226.
Acknowledgements
The authors thank the patients and their family members for the participation in this study. We thank PGIMER, Chandigarh, for financial support.
Funding
The study was financially supported by the PGIMER, Chandigarh [No.71/2-Edu-16/4484] through intramural research grant scheme. M.K. was funded by University Grants Commission (UGC) fellowship. T.S. was funded by a PGIMER fellowship. K.P. was funded by CSIR, New Delhi.
Author information
Authors and Affiliations
Contributions
Conceptualization: HB, M Kumar, AC, HG, KKM, VKG; Sample and data collection: M Kumar, SC, TS; Samples provided by: AA, NS, M Karthigeyan, AS, SKS, MT, AT, YSB, SKG, KKM; Research facilities: TG, AP, SVA, RKR; Bioinformatics analysis: KP; Data Interpretation: M Kumar, HB; Project administration: HB; Supervision: HB, VKG, RKV, RP, M Khullar, AC, HG; Validation: M Kumar, TS; Visualization: M Kumar, TS, KP; Writing-original draft preparation: M Kumar, HB; Writing-review and editing: M Kumar, HB, KP, TS, RP, AC, HG. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study was approved by the Institute Ethics Committee of PGIMER, Chandigarh, India (IEC no. MK/2866/Ph.D/7735). All the patients/subjects were enrolled only after obtaining the written informed consent from patients/relatives.
Consent for Publication
NA
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Munish Kumar is the first author.
Rakesh Kumar Vashishta, Kanchan Kumar Mukherjee and Vinod Kumar are deceased.
Supplementary Information
ESM 1
Supplementary Table 1. List of Primers used for qRT-PCR. Supplementary Table 2: Clinicopathological details of study participants. Supplementary Table 3. RNA Integrity Number (RIN) of samples. Supplementary Table 4. Complete list of significantly dysregulated genes between Unruptured IA and Control. Supplementary Table 5. Complete list of significantly dysregulated genes between Ruptured IA and Unruptured IA (PDF 165 kb)
ESM 2
Supplementary Figure 1. Principal Component Analysis. A. Unruptured IA (T1) vs Control (C). B. Ruptured IA (T2) vs Unruptured IA (T1). Supplementary Figure 2. Protein-Protein Interaction network - Unruptured IA vs Control. Supplementary Figure 3. Protein-Protein Interaction network - Ruptured IA vs Unruptured IA (PDF 625 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, M., Patel, K., Chinnapparaj, S. et al. Dysregulated Genes and Signaling Pathways in the Formation and Rupture of Intracranial Aneurysm. Transl. Stroke Res. (2023). https://doi.org/10.1007/s12975-023-01178-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12975-023-01178-w