Abstract
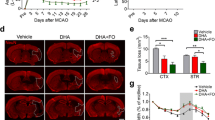
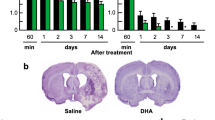
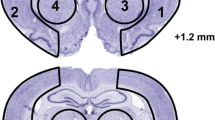
Prophylactic dietary intake of omega-3 polyunsaturated fatty acids (n-3 PUFAs) has been shown to remarkably ameliorate ischemic brain injury. However, the therapeutic efficacy of n-3 PUFA administration post-stroke, especially its impact on neurovascular remodeling and long-term neurological recovery, has not been fully characterized thus far. In this study, we investigated the effect of n-3 PUFA supplementation, as well as in combination with docosahexaenoic acid (DHA) injections, on long-term stroke outcomes. Mice were subjected to transient middle cerebral artery occlusion (MCAO) before randomly assigned to four groups to receive the following: (1) low dose of n-3 PUFAs as the vehicle control, (2) intraperitoneal DHA injections, (3) n-3 PUFA dietary supplement, or (4) combined treatment of (2) and (3). Neurological deficits and brain atrophy, neurogenesis, angiogenesis, and glial scar formation were assessed up to 28 days after MCAO. Results revealed that groups 2 and 3 showed only marginal reduction in post-stroke tissue loss and attenuation of cognitive deficits. Interestingly, group 4 exhibited significantly reduced tissue atrophy and improved cognitive functions compared to groups 2 and 3 with just a single treatment. Mechanistically, the combined treatment promoted post-stroke neurogenesis and angiogenesis, as well as reduced glial scar formation, all of which significantly correlated with the improved spatial memory in the Morris water maze. These results demonstrate an effective therapeutic regimen to enhance neurovascular restoration and long-term cognitive recovery in the mouse model of MCAO. Combined post-stroke DHA treatment and n-3 PUFA dietary supplementation thus may be a potential clinically translatable therapy for stroke or related brain disorders.






Similar content being viewed by others
References
Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020–35.
Lapchak PA. Critical early thrombolytic and endovascular reperfusion therapy for acute ischemic stroke victims: a call for adjunct neuroprotection. Transl Stroke Res. 2015;6(5):345–54.
Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl Stroke Res. 2014;5(4):442–53.
Hafez S, Hoda MN, Guo X, Johnson MH, Fagan SC, Ergul A. Comparative analysis of different methods of ischemia/reperfusion in hyperglycemic stroke outcomes: interaction with tPA. Transl Stroke Res. 2015;6(3):171–80.
Mandava P, Shah SD, Sarma AK, Kent TA. An outcome model for intravenous rt-PA in acute ischemic stroke. Transl Stroke Res. 2015;6(6):451–7.
Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11(4):369–80.
Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, et al. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol. 2014;115:138–56.
Ruan L, Lau BW, Wang J, Huang L, Zhuge Q, Wang B, et al. Neurogenesis in neurological and psychiatric diseases and brain injury: from bench to bedside. Prog Neurobiol. 2014;115:116–37.
Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, et al. Cell based therapies for ischemic stroke: from basic science to bedside. Prog Neurobiol. 2014;115:92–115.
Gupta S. Brain food: clever eating. Nature. 2016;531(7592):S12–3.
Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365–79.
Zhang M, Wang S, Mao L, Leak RK, Shi Y, Zhang W, et al. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J Neurosci. 2014;34(5):1903–15.
Belayev L, Khoutorova L, Atkins KD, Bazan NG. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009;40(9):3121–6.
Lalancette-Hebert M, Julien C, Cordeau P, Bohacek I, Weng YC, Calon F, et al. Accumulation of dietary docosahexaenoic acid in the brain attenuates acute immune response and development of postischemic neuronal damage. Stroke. 2011;42(10):2903–9.
Shi Z, Ren H, Luo C, Yao X, Li P, He C et al. Enriched endogenous omega-3 polyunsaturated fatty acids protect cortical neurons from experimental ischemic injury. Mol Neurobiol. 2015. [Epub ahead of print]
Hong SH, Khoutorova L, Bazan NG, Belayev L. Docosahexaenoic acid improves behavior and attenuates blood-brain barrier injury induced by focal cerebral ischemia in rats. Exp Transl Stroke Med. 2015;7(1):3.
Zhang W, Zhang H, Mu H, Zhu W, Jiang X, Hu X, et al. Omega-3 polyunsaturated fatty acids mitigate blood-brain barrier disruption after hypoxic-ischemic brain injury. Neurobiol Dis. 2016;91:37–46.
Wang J, Shi Y, Zhang L, Zhang F, Hu X, Zhang W, et al. Omega-3 polyunsaturated fatty acids enhance cerebral angiogenesis and provide long-term protection after stroke. Neurobiol Dis. 2014;68:91–103.
Zhang W, Wang H, Zhang H, Leak RK, Shi Y, Hu X, et al. Dietary supplementation with omega-3 polyunsaturated fatty acids robustly promotes neurovascular restorative dynamics and improves neurological functions after stroke. Exp Neurol. 2015;272:170–80.
Hu X, Zhang F, Leak RK, Zhang W, Iwai M, Stetler RA, et al. Transgenic overproduction of omega-3 polyunsaturated fatty acids provides neuroprotection and enhances endogenous neurogenesis after stroke. Curr Mol Med. 2013;13(9):1465–73.
Shi Y, Zhang L, Pu H, Mao L, Hu X, Jiang X, et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun. 2016;7:10523.
Han L, Cai W, Mao L, Liu J, Li P, Leak RK, et al. Rosiglitazone promotes white matter integrity and long-term functional recovery after focal cerebral ischemia. Stroke. 2015;46(9):2628–36.
Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200.
Springer J, Schust S, Peske K, Tschirner A, Rex A, Engel O, et al. Catabolic signaling and muscle wasting after acute ischemic stroke in mice: indication for a stroke-specific sarcopenia. Stroke. 2014;45(12):3675–83.
Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57(2):202–7.
Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24(7):805–13.
Stetler RA, Cao G, Gao Y, Zhang F, Wang S, Weng Z, et al. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008;28(49):13038–55.
Chen Q, Zhang J, Guo J, Tang J, Tao Y, Li L, et al. Chronic hydrocephalus and perihematomal tissue injury developed in a rat model of intracerebral hemorrhage with ventricular extension. Transl Stroke Res. 2015;6(2):125–32.
Zuloaga KL, Zhang W, Yeiser LA, Stewart B, Kukino A, Nie X, et al. Neurobehavioral and imaging correlates of hippocampal atrophy in a mouse model of vascular cognitive impairment. Transl Stroke Res. 2015;6(5):390–8.
Wang G, Jiang X, Pu H, Zhang W, An C, Hu X, et al. Scriptaid, a novel histone deacetylase inhibitor, protects against traumatic brain injury via modulation of PTEN and AKT pathway: scriptaid protects against TBI via AKT. Neurotherapeutics. 2013;10(1):124–42.
Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–61.
Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–34.
Zhao S, Qu H, Zhao Y, Xiao T, Zhao M, Li Y, et al. CXCR4 antagonist AMD3100 reverses the neurogenesis and behavioral recovery promoted by forced limb-use in stroke rats. Restor Neurol Neurosci. 2015;33(6):809–21.
Kuptsova K, Kvist E, Nitzsche F, Jolkkonen J. Combined enriched environment/atipamezole treatment transiently improves sensory functions in stroke rats independent from neurogenesis and angiogenesis. Rom J Morphol Embryol. 2015;56(1):41–7.
Zhang W, Cheng J, Vagnerova K, Ivashkova Y, Young J, Cornea A, et al. Effects of androgens on early post-ischemic neurogenesis in mice. Transl Stroke Res. 2014;5(2):301–11.
Pena I, Borlongan CV. Translating G-CSF as an adjunct therapy to stem cell transplantation for stroke. Transl Stroke Res. 2015;6(6):421–9.
Okada M, Nakanishi H, Tamura A, Urae A, Mine K, Yamamoto K, et al. Long-term spatial cognitive impairment after middle cerebral artery occlusion in rats: no involvement of the hippocampus. J Cereb Blood Flow Metab. 1995;15(6):1012–21.
Yonemori F, Yamaguchi T, Yamada H, Tamura A. Spatial cognitive performance after chronic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19(5):483–94.
Block F, Kunkel M, Schwarz M. Quinolinic acid lesion of the striatum induces impairment in spatial learning and motor performance in rats. Neurosci Lett. 1993;149(2):126–8.
Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8(5):491–500.
Wang L, Wang X, Su H, Han Z, Yu H, Wang D, et al. Recombinant human erythropoietin improves the neurofunctional recovery of rats following traumatic brain injury via an increase in circulating endothelial progenitor cells. Transl Stroke Res. 2015;6(1):50–9.
Soliman S, Ishrat T, Fouda AY, Patel A, Pillai B, Fagan SC. Sequential therapy with minocycline and candesartan improves long-term recovery after experimental stroke. Transl Stroke Res. 2015;6(4):309–22.
Li Q, Khatibi N, Zhang JH. Vascular neural network: the importance of vein drainage in stroke. Transl Stroke Res. 2014;5(2):163–6.
Zhang JH. Vascular neural network in subarachnoid hemorrhage. Transl Stroke Res. 2014;5(4):423–8.
Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, et al. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016;131(3):323–45.
Chen D, Yu SP, Wei L. Ion channels in regulation of neuronal regenerative activities. Transl Stroke Res. 2014;5(1):156–62.
Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29(37):11511–22.
Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–56.
He K, Rimm EB, Merchant A, Rosner BA, Stampfer MJ, Willett WC, et al. Fish consumption and risk of stroke in men. JAMA. 2002;288(24):3130–6.
Mohajeri MH, Troesch B, Weber P. Inadequate supply of vitamins and DHA in the elderly: implications for brain aging and Alzheimer-type dementia. Nutrition. 2015;31(2):261–75.
King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci. 2006;26(17):4672–80.
Eady TN, Khoutorova L, Anzola DV, Hong SH, Obenaus A, Mohd-Yusof A, et al. Acute treatment with docosahexaenoic acid complexed to albumin reduces injury after a permanent focal cerebral ischemia in rats. PLoS One. 2013;8(10):e77237.
Eady TN, Khoutorova L, Obenaus A, Mohd-Yusof A, Bazan NG, Belayev L. Docosahexaenoic acid complexed to albumin provides neuroprotection after experimental stroke in aged rats. Neurobiol Dis. 2014;62:1–7.
Eady TN, Khoutorova L, Atkins KD, Bazan NG, Belayev L. Docosahexaenoic acid complexed to human albumin in experimental stroke: neuroprotective efficacy with a wide therapeutic window. Exp Transl Stroke Med. 2012;4(1):19.
Hong SH, Belayev L, Khoutorova L, Obenaus A, Bazan NG. Docosahexaenoic acid confers enduring neuroprotection in experimental stroke. J Neurol Sci. 2014;338(1-2):135–41.
Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukot Essent Fatty Acids. 2009;80(2-3):157–63.
Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52.
Sumiyoshi M, Satomi J, Kitazato KT, Yagi K, Shimada K, Kurashiki Y, et al. PPARgamma-dependent and -independent inhibition of the HMGB1/TLR9 pathway by eicosapentaenoic acid attenuates ischemic brain damage in ovariectomized rats. J Stroke Cerebrovasc Dis. 2015;24(6):1187–95.
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–70.
Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110(4):429–41.
Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–94.
Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–47.
Rossi D. Astrocyte physiopathology: at the crossroads of intercellular networking, inflammation and cell death. Prog Neurobiol. 2015;130:86–120.
Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28(28):7231–43.
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24(9):2143–55.
Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44.
Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390(6661):680–3.
An C, Shi Y, Li P, Hu X, Gan Y, Stetler RA, et al. Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog Neurobiol. 2014;115:6–24.
Hu X, Liou AK, Leak RK, Xu M, An C, Suenaga J et al. Neurobiology of microglial action in CNS injuries: receptor-mediated signaling mechanisms and functional roles. Prog Neurobiol. 2014;119-120:60-84.
Seifert HA, Pennypacker KR. Molecular and cellular immune responses to ischemic brain injury. Transl Stroke Res. 2014;5(5):543–53.
Franco R, Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:65–86.
Li L, Tao Y, Tang J, Chen Q, Yang Y, Feng Z, et al. A cannabinoid receptor 2 agonist prevents thrombin-induced blood-brain barrier damage via the inhibition of microglial activation and matrix metalloproteinase expression in rats. Transl Stroke Res. 2015;6(6):467–77.
Mallucci G, Peruzzotti-Jametti L, Bernstock JD, Pluchino S. The role of immune cells, glia and neurons in white and gray matter pathology in multiple sclerosis. Prog Neurobiol. 2015;127-128:1-22.
Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, et al. Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol. 2014;117:54–72.
Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317.
Jin R, Zhu X, Li G. Embolic middle cerebral artery occlusion (MCAO) for ischemic stroke with homologous blood clots in rats. J Vis Exp. 2014;91:51956.
Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766(1-2):83–92.
Acknowledgments
*H.P. and *X.J. contributed equally to this research. This project was supported by the US Department of Veterans Affairs (VA) RR&D Merit Review RX000420; the US National Institutes of Health grants NS045048, NS091175, and NS095671; the American Heart Association grant 13SDG14570025; and the Chinese Natural Science Foundation grants 81529002, 81171149, 81371306, 81571285, and 81100978. J.C. is a recipient of the VA Senior Research Career Scientist Award. The authors are indebted to Pat Strickler for excellent administrative support. The present address of J.X. is Cerebrovascular Center, Henan Provincial People’s Hospital, Zhengzhou University, Zhengzhou 450003, China.
Authors’ Contributions
Y.S., Y.G., X.H., and J.C. designed the research. H.P., X.J., J.X., and W.Z. performed the research. X.J. and Y.S. analyzed the data. H.P., X.J., D.H., J.C., and Y.S. wrote the manuscript. All authors reviewed and edited the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Hongjian Pu and Xiaoyan Jiang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Post-stroke DHA and FO treatments do not change hippocampal neurogenesis in the non-injured contralateral hemisphere. a Representative images of double-label immunostaining of BrdU (green) and NeuN (red) in the hippocampal CA1, CA3 and DG areas of the non-injured contralateral hemisphere at 28 days after MCAO. There was barely any BrdU immunosignal in all regions. Scale bar: 50 μm. b-d Quantification of total cells that are positive for NeuN immunosignal in CA1, CA3, and DG of the contralateral hippocampus. There was no statistical difference among all groups. e-g Quantification of BrdU+/NeuN+ cells in CA1, CA3, and DG of the contralateral hippocampus. Few BrdU+/NeuN+ cells were observed in all regions. There was no statistical difference among all groups. n = 7 mice per group. (DOCX 2039 kb)
Rights and permissions
About this article
Cite this article
Pu, H., Jiang, X., Hu, X. et al. Delayed Docosahexaenoic Acid Treatment Combined with Dietary Supplementation of Omega-3 Fatty Acids Promotes Long-Term Neurovascular Restoration After Ischemic Stroke. Transl. Stroke Res. 7, 521–534 (2016). https://doi.org/10.1007/s12975-016-0498-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-016-0498-y