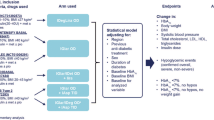
Abstract
Introduction
iGlarLixi is a titratable, fixed-ratio combination of insulin glargine (iGlar, 100 units/ml) and the glucagon-like peptide-1 receptor agonist lixisenatide for the treatment of patients with type 2 diabetes. This post hoc analysis of the phase 3 LixiLan-L trial (NCT02058160) investigated baseline characteristics, glycemic control, and safety outcomes in participants who received the study-specified maximum dose (60 units/day) of iGlarLixi or iGlar vs. those who received < 60 units/day.
Methods
Outcomes were compared for participants receiving 60 or < 60 units/day at week 30. Endpoints analyzed included change in A1C, fasting plasma glucose (FPG), 2-h postprandial glucose (2-h PPG), body weight, proportion of participants achieving A1C < 7.0%, proportion of participants receiving rescue therapy, documented symptomatic hypoglycemia, and gastrointestinal adverse event (GI AE) incidence.
Results
By week 30, 27% (iGlarLixi) and 31% (iGlar) of participants received the maximum dose. Participants on 60 vs. < 60 units/day were younger and had higher body weight, body mass index (BMI), FPG, and baseline insulin dose. In both dose groups, A1C change from baseline was significantly greater with iGlarLixi vs. iGlar, and more participants treated with iGlarLixi vs. iGlar achieved A1C < 7.0%. No significant differences were observed in change from baseline for A1C, FPG, 2-h PPG, or GI AE incidence between insulin dose groups, regardless of treatment. In both treatment arms, incidence of symptomatic hypoglycemia was lower in participants receiving 60 units/day vs. those receiving < 60 units/day. Participants treated with iGlarLixi (< 60 or 60 units/day) had modest weight loss over 30 weeks vs. an increase in weight compared with iGlar.
Conclusions
Maximum doses of iGlarLixi were required in participants with a more insulin-resistant clinical phenotype (younger, higher BMI, FPG, and insulin doses). Benefits were observed with iGlarLixi vs. iGlar, even at 60 units/day, with more participants achieving glycemic goals, no increase in symptomatic hypoglycemia, and a modest reduction in body weight.
Funding
Sanofi US, Inc.
Similar content being viewed by others
Introduction
Despite widespread acceptance of the importance of good glycemic control in lowering the risk of micro- and macrovascular diabetes complications, many patients with poorly controlled type 2 diabetes (T2D) experience significant delay of up to 7 years or more after the second oral therapy before starting basal insulin therapy [1] and an additional 3.7 years for further intensification [1,2,3,4]. Diabetes impacts multiple systems within the body, and achievement and maintenance of glycemic goals are important in preventing or at least delaying the development and progression of diabetes-associated complications [5]. To effectively treat T2D, treatment intensification is often required using combinations of medications that address one or more of the many pathologic processes associated with the disease [6]. Combination therapy using the complementary mechanisms of action of a basal insulin and a glucagon-like peptide-1 receptor agonist (GLP-1 RA) targets seven of the many pathophysiologic defects in T2D, addressing both fasting plasma glucose (FPG) and postprandial glucose (PPG) levels to effectively improve glycemic control in patients with T2D compared with treatment with either basal insulin or a GLP-1 RA alone [6,7,8].
iGlarLixi is a once-daily, titratable, fixed-ratio combination of insulin glargine 100 units/ml (iGlar) and the GLP-1 RA lixisenatide (lixisenatide, 33 µg/ml), currently approved in the US as an adjunct to diet and exercise to improve glycemic control in adults with T2D [9] and in the EU for patients uncontrolled on metformin alone, metformin combined with another oral antidiabetes drug (OAD), and patients uncontrolled on basal insulin [10]. The phase 3 LixiLan-L and LixiLan-O clinical trials have shown that both insulin-experienced and -naive patients treated with iGlarLixi had significantly greater reductions in glycated hemoglobin (A1C) and were more likely to achieve A1C < 7.0% (< 53 mmol/mol) than patients receiving either iGlar or lixisenatide alone [11, 12]. These studies also demonstrated that simultaneous administration and slow up-titration of iGlarLixi mitigated gastrointestinal adverse events (GI AEs) compared with lixisenatide alone, and weight gain compared with iGlar alone, and that rates of hypoglycemia were similar to iGlar alone [11, 12]. In the LixiLan-L trial [11], iGlarLixi had a maximum insulin dose of 60 units/day to ensure that the dose of the lixisenatide component did not exceed the recommended dose of 20 µg/day; the dose of iGlar in the comparison arm was also capped at 60 units/day to provide an equal basal insulin comparison and assess the impact of lixisenatide to the overall effect of iGlarLixi. It was anticipated that a maximum insulin dose of 60 units/day would allow most patients to achieve their glycemic target since the average dose of basal insulin analogs has been reported to be around 30–40 units/day [13, 14]. However, many patients do not attain or maintain their target A1C with basal insulin alone. In this post hoc analysis, it was hypothesized that the clinical characteristics of those participants requiring the maximum dose of 60 units/day in either treatment group may be different from those who did not and that even at the maximum dose of 60 units/day there would be glycemic benefit with iGlarLixi compared with iGlar.
The objectives of this post hoc analysis of data from the LixiLan-L trial were to explore the baseline characteristics of participants who received the maximum dose of 60 units/day vs. those who received < 60 units/day and compare glycemic and safety outcomes of iGlarLixi vs. iGlar at week 30 within and between each dose group.
Methods
Study Design
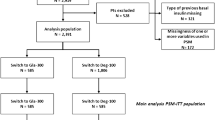
The LixiLan-L trial (NCT02058160) was a phase 3, randomized, open-label, parallel-group study that investigated the efficacy and safety of iGlarLixi in participants with T2D uncontrolled on basal insulin with or without up to two OADs. The full details of the trial have been published previously [11]. Adult participants were eligible for study enrollment if they had been diagnosed with T2D at least 1 year before screening, had been on basal insulin for at least 6 months before screening, and had a stable basal insulin regimen with doses of 15–40 units/day (± 20%) for at least 2 months prior to the screening visit. During a 6-week run-in phase, any OADs other than metformin were discontinued; participants were switched to iGlar (if previously on another basal insulin), and the daily dose of iGlar was titrated/optimized for all participants to achieve FPG ≤ 140 mg/dl. After the run-in phase, eligible participants [A1C level of 7–10% (53–86 mmol/mol), mean fasting self-measured plasma glucose (SMPG) of ≤ 140 mg/dl, and iGlar dose of 20–50 units/day] were randomized in a 1:1 ratio to receive once-daily open-label treatment with iGlarLixi or iGlar for 30 weeks. Mean A1C at screening was 8.5% (69 mmol/mol) for both iGlarLixi and iGlar participants, which decreased during the 6-week run-in phase to 8.1% for both groups. Both iGlarLixi and iGlar could be titrated by up to 4 units per week to attain and maintain a target fasting SMPG of 80–100 mg/dl while avoiding hypoglycemia; the dose of both iGlarLixi and iGlar was capped at 60 units/day. Rescue medication (insulin glulisine) was introduced along with iGlarLixi or iGlar on a background of metformin (if taken) as a single daily injection at the main meal if FPG values were > 240 mg/dl (weeks 8–11) or > 200 mg/dl (weeks 12–30) over 3 consecutive days. No other oral or injectable antidiabetic treatment was permitted as rescue medication in either treatment group. For this post hoc analysis, participants in the LixiLan-L trial were subdivided into two groups based on their insulin dose at week 30 (< 60 units/day and 60 units/day). As previously reported [11], the LixiLan-L trial was designed and monitored in accordance with Good Clinical Practice, the International Conference on Harmonization, and the Declaration of Helsinki. Institutional review boards or ethics committees at each study site approved the protocol. Each patient gave written informed consent.
Efficacy and Safety Endpoints
The primary endpoint of this post hoc analysis was the change in A1C from baseline to end of study [week 30 or last observation carried forward (LOCF)] within and between treatment groups and dose groups (< 60 U or 60 U). Secondary endpoints included FPG, 2-h PPG, body weight, 7-point SMPG profile, insulin dose by body weight (≤ 0.5 units/kg or > 0.5 units/kg), proportion of participants achieving A1C goal [< 7.0% (< 53 mmol/mol)], and the proportion of participants reaching A1C < 7.0% (< 53 mmol/mol) at week 30 with no weight gain or hypoglycemia. Safety endpoints included exposure-adjusted rates of documented symptomatic hypoglycemia [defined as typical symptoms of hypoglycemia accompanied by an SMPG value of ≤ 70 mg/dl (≤ 3.9 mmol/l)] and incidence of GI AEs (nausea, vomiting, and diarrhea).
Statistical Analysis
This post hoc analysis involved a modified intent-to-treat population consisting of all randomized participants with both baseline and study end (LOCF) measurements that were used for efficacy measures. The safety population consisted of all randomized participants who received at least one dose of iGlarLixi or iGlar, regardless of the treatment dose administered. Data are presented descriptively [number (n), mean, and standard deviation (SD)] by treatment group and were analyzed using a two-sample t test for continuous variables, Pearson chi-squared (χ2) tests for proportions, and Fisher exact tests for race proportions because of the low patient numbers in the subgroups. Data are also presented stratified by final daily insulin dose per kg body weight (≤ 0.5 units/kg and > 0.5 units/kg). In the predictor analysis for participants reaching 60 units/day, the regression covariates included age, baseline body mass index (BMI), baseline FPG, and baseline dose. In the analysis of participants achieving the A1C goal of < 7.0% (< 53 mmol/mol), the regression covariates included treatment arm and baseline A1C. Predictor analyses were carried out using logistic stepwise regression analyses to control for key patient baseline characteristics and assessed the outcomes of reaching the maximum dose of 60 units/day and the glycemic goal of A1C < 7.0% (< 53 mmol/mol). Tipping point analyses were used to compare variable relationships and the potential effect of dose capping at 60 units/day. A p value of 0.05 was used to indicate statistical significance.
Results
Patient Demographics and Baseline Characteristics
Overall, 336/366 (92%) of patients in the iGlarLixi group and 355/365 (96%) in the iGlar group completed treatment. Full details of patient disposition have been described previously [11].
After 30 weeks, 27.0% (99/366) of participants in the iGlarLixi arm reached the maximum dose of 60 units/day compared with 30.7% (112/365) in the iGlar arm (Tables 1, 2). In the iGlarLixi arm, participants who received the maximum dose of 60 units/day at week 30 were younger and had a higher baseline body weight, BMI, A1C, FPG, insulin dose, and insulin dose by weight compared with participants at < 60 units/day iGlarLixi (Table 1). There were no statistically significant differences between dose groups regarding sex, race, duration of diabetes, or baseline 2-h PPG levels. In the iGlar treatment arm, participants who received doses of 60 units/day at week 30 were younger, had a shorter duration of diabetes, and a higher baseline body weight, BMI, FPG, insulin dose, and insulin dose by weight compared with participants receiving < 60 units/day iGlar. There were no statistically significant differences between dose groups regarding sex, race, baseline A1C, or baseline 2-h PPG levels (Table 1).
There was no significant difference in baseline characteristics between participants who received 60 units/day iGlarLixi vs. 60 units/day iGlar or who received < 60 units/day iGlarLixi vs. < 60 units/day iGlar, except for BMI, which was significantly higher in the 60 units/day iGlarLixi group vs. the 60 units/day iGlar group (33.8 vs. 32.3, respectively; p = 0.0003).
Treatment Outcomes
Efficacy Endpoints
Overall, patients treated with iGlarLixi vs. iGlar in both dose groups showed significantly greater reductions from baseline in A1C [− 1.2% vs. − 0.6% (− 6.1 vs. − 3.0 mmol/mol) in participants receiving < 60 units/day and − 1.0% vs. − 0.5% (− 5.0 vs. − 2.5 mmol/mol) in participants receiving 60 units/day, respectively (p < 0.0001 for both)] (Table 3). In both treatment arms, A1C reductions from baseline were similar for participants treated with doses of < 60 units/day and 60 units/day (p = 0.1233 and p = 0.0935, respectively) (Table 4). Final A1C levels were lower with iGlarLixi vs. iGlar in both participants who received < 60 units/day (p < 0.0001) and those who received 60 units/day (p = 0.0003). Final A1C levels were significantly lower in participants who received < 60 units/day in both treatment groups (iGlarLixi p = 0.0009; iGlar p = 0.0169). In addition, more participants treated with iGlarLixi achieved A1C < 7.0% (< 53 mmol/mol) compared with iGlar regardless of dose at week 30 (Table 2; Fig. 1a). In both treatment arms, more participants treated with < 60 units/day achieved A1C < 7.0% (< 53 mmol/mol) compared with those receiving 60 units/day (p = 0.0016 for iGlarLixi 60 units/day vs. < 60 units/day; p = 0.0698 for iGlar 60 units/day vs. < 60 units/day).
Percentage of patients using < 60 units/day or 60 units/day of iGlarLixi/iGlar achieving a glycated hemoglobin < 7.0% (< 53 mmol/mol) at week 30 and b glycated hemoglobin < 7.0% (< 53 mmol/mol) with no body weight gain at week 30 and with no hypoglycemia during the study. Analyses based on safety population. A1C glycated hemoglobin, iGlar insulin glargine 100 units/ml, iGlarLixi fixed-ratio combination of iGlar and lixisenatide
More participants treated with iGlarLixi vs. iGlar in both dose groups achieved A1C < 7.0% (< 53 mmol/mol) without weight gain or documented symptomatic hypoglycemia (60 units/day: p = 0.0019; < 60 units/day: p = 0.0007) (Table 3; Fig. 1b). In both treatment arms, fewer participants receiving 60 units/day achieved this composite endpoint compared with those receiving < 60 units/day. However, these differences were statistically significant for iGlar only (p = 0.0441) (Table 4; Fig. 1b). As would be expected given its mode of action, change from baseline in 2-h PPG was greater with iGlarLixi compared with iGlar in both dose groups (p < 0.0001 for both comparisons) (Table 3; Fig S1 in the electronic supplementary material). There were no significant differences in FPG or 2-h PPG change from baseline between participants treated with doses of < 60 units/day or 60 units/day in either treatment arm (Fig. S1 in the electronic supplementary material; Table 4). Participants treated with iGlarLixi in both dose groups showed a decrease in body weight at week 30 compared with a gain in participants in both iGlar dose groups (p = 0.0003 for iGlarLixi 60 units/day vs. iGlar 60 units/day; p < 0.0001 for iGlarLixi < 60 units/day vs. iGlar < 60 units/day) (Fig. 2a). There was no significant difference between change in body weight in the < 60 units/day group compared with the 60 units/day group with iGlarLixi (p = 0.1489) (Table 4; Fig. 2a). In the iGlar arm, weight gain was significantly greater in the 60 units/day group compared with the < 60 units/day group (p = 0.0254) (Fig. 2a).
Rescue Therapy
In the LixiLan-L trial, 55 participants (15.0%) treated with iGlarLixi reached the maximum dose of 60 units/day, but did not reach the A1C goal compared with 85 participants (23.3%) in the iGlar arm (Table S1a in the electronic supplementary material). Of the participants who reached 60 units/day, 4 participants in the iGlarLixi arm received rescue therapy compared with 12 in the iGlar arm (p = 0.2812). There were no significant differences in the numbers of participants receiving rescue therapy between dosing subgroups (iGlarLixi 60 units/day vs. < 60 units/day, p = 0.0976; iGlar 60 units/day vs. < 60 units/day, p = 0.1690). Time to rescue was 170 days in the iGlarLixi arm and 120 days in the iGlar arm. Baseline characteristics of the participants who reached 60 units/day and received rescue therapy are presented in Table S1b in the electronic supplementary material.
Safety Endpoints
There was no significant difference between the rate of documented symptomatic hypoglycemia between iGlarLixi and iGlar in either dose group (Tables 3, 4; Fig. 2b). In both treatment arms, the incidence of documented symptomatic hypoglycemia was lower in participants receiving 60 units/day than in those receiving < 60 units/day (Fig. 2b). In the iGlarLixi arm, 28.3% of participants receiving 60 units/day experienced documented symptomatic hypoglycemia compared with 44.4% of those receiving < 60 units/day (p = 0.0053). Similarly, in the iGlar arm, 29.5% of participants receiving 60 units/day reported documented symptomatic hypoglycemia compared with 48.2% of participants receiving < 60 units/day (p = 0.0008).
Overall, fewer participants experienced GI AEs in the iGlar arm compared with those in the iGlarLixi arm (4.7% vs. 12.8% in the < 60 units/day group, respectively; p = 0.0017; 0.9% vs. 11.1% in the 60 units/day group, respectively; p = 0.0016) (Table 3). The incidence of GI AEs was similar for participants receiving < 60 units/day and 60 units/day in the iGlarLixi arm (12.8% vs. 11.1%, respectively), but was higher for participants receiving < 60 units/day than those receiving 60 units/day in the iGlar arm (4.7% vs. 0.9%, respectively) (Table 4).
Analysis by Final Daily Basal Insulin Dose per Kilogram Body Weight
Clinical experience as well as recent analyses of insulin glargine U100 have indicated that when a basal insulin dose of 0.5 units/kg/day is approached or exceeded, there is little incremental glycemic benefit with the disadvantage of weight gain [15]. The percentages of participants in this analysis who reached an A1C goal of < 7.0% (< 53 mmol/mol) were comparable, regardless of whether participants received a dose of ≤ 0.5 units/kg/day or > 0.5 units/kg/day insulin in both the iGlarLixi and iGlar arms (iGlarLixi: 56.8% vs. 56.3%, respectively, p = 0.9235; iGlar: 32.7% vs. 29.5%, respectively, p = 0.5203) (Table S2 in the electronic supplementary material). However, participants receiving ≤ 0.5 units/kg/day of iGlarLixi demonstrated significantly greater weight loss than those receiving > 0.5 units/kg/day (− 1.2 kg vs. − 0.2 kg, respectively; p < 0.0070). Interestingly, weight gain from baseline to study end was comparable between participants receiving ≤ 0.5 units/kg/day or > 0.5 units/kg/day insulin in the iGlar arm (0.6 kg vs. 0.9 kg, respectively; p = 0.3154 (Table S2 in the electronic supplementary material). The incidence of documented symptomatic hypoglycemia was numerically higher for comparable participants receiving ≤ 0.5 units/kg/day vs. > 0.5 units/kg/day of either iGlar (46.4% vs. 40.0%, respectively; p = 0.2231) or iGlarLixi (44.5% vs. 37.6%, respectively; p = 0.1867) (Table S2 in the electronic supplementary material). The incidence of GI AEs was numerically lower with doses ≤ 0.5 units/kg/day vs. > 0.5 units/kg/day (iGlar: 2.0% vs. 4.8%, respectively, p = 0.2520; iGlarLixi: 8.9% vs. 14.1%, respectively, p = 0.1851) (Table S2 in the electronic supplementary material).
Predictor Analyses
Stepwise logistic regression analyses indicated that baseline characteristics that were statistically significant predictors of participants reaching 60 units/day were basal insulin dose (p < 0.0001), BMI (p < 0.0001), age (p = 0.0003), and FPG (p = 0.0001). Duration of T2D and baseline weight were not predictors of participants reaching 60 units/day in the regression analysis after adjusting for multiple variables. Significant predictors of participants achieving A1C goals of < 7.0% (< 53 mmol/mol) were baseline A1C levels (p < 0.0001) and treatment with iGlarLixi vs. iGlar (p < 0.0001). Baseline basal insulin dose and patient age were not predictors of achieving glycemic goals.
Tipping Point Analysis
Higher baseline BMI and higher baseline insulin dose were associated with a greater likelihood of reaching 60 units/day in both treatment arms. iGlarLixi participants with a baseline BMI of > 42 kg/m2 (Pearson correlation coefficient (r), r = 0.4213, p < 0.0001) and those receiving baseline insulin doses of ≥ 0.78 units/kg/day (iGlarLixi: r = 0.3255, p < 0.0001) were more likely to reach 60 units/day than those receiving < 42 kg/m2 or < 0.78 units/kg/day. In the iGlar arm, participants with a baseline BMI of > 45 kg/m2 (iGlar: r = 0.3047, p < 0.0001) or baseline insulin dose ≥ 0.70 units/kg/day (iGlar: r = 0.4042, p < 0.0001) were more likely to reach 60 units/day than those participants with values below these thresholds. (Fig. S2a and S2b in the electronic supplementary material). Tipping point analyses by quartiles suggested that baseline BMI (Fig. S3a in the electronic supplementary material) (iGlarLixi: p = 0.4199; iGlar: p = 0.3033) and baseline dose/kg (Fig. S3b in the electronic supplementary material) (iGlarLixi: p = 0.1032; iGlar: p = 0.1030) had little effect on final A1C levels in either treatment arm.
Discussion
In this post hoc analysis of patients with T2D who participated in the LixiLan-L trial, patient characteristics associated with greater insulin resistance (including age, body weight, BMI, A1C at baseline, and FPG at baseline) tended to predict participants who would require the maximum dose of 60 units/day. Irrespective of the final daily insulin dose, iGlarLixi, compared with iGlar, led to greater A1C reductions and a higher percentage of participants achieving A1C < 7.0% (< 53 mmol/mol) and the composite endpoint of A1C < 7.0% (< 53 mmol/mol) with no documented hypoglycemia and no weight gain. In addition, there was no significant difference in change from baseline for A1C, FPG, or 2-h PPG between participants who required < 60 units/day or 60 units/day, indicating that iGlarLixi was similarly effective in the minority of participants who required titration to maximum insulin dose and the majority who did not.
In the current analysis, participants receiving 60 units/day of either iGlarLixi or iGlar experienced a lower incidence of documented symptomatic hypoglycemia compared with those receiving < 60 units/day. This may appear to be counterintuitive given that greater insulin doses are generally considered to be associated with increased rates of hypoglycemia. Our findings may reflect the difference in patient phenotype in the higher and lower dose participants in LixiLan-L shown in the current analysis. Participants who required the maximum dose of iGlarLixi or iGlar tended to have characteristics associated with greater insulin resistance. This implies that more insulin-sensitive (and thus more hypoglycemia-prone) participants required less insulin, while the more insulin-resistant (greater BMI, younger, higher FPG, and baseline insulin dose) required higher doses to achieve glycemic control, but experienced less hypoglycemia because of their relative insulin resistance. Alternatively, this finding may correspond to hypoglycemia as a barrier to up-titration for both iGlarLixi and iGlar in the LixiLan-L trial [11]. When a patient’s A1C remains above target despite their FPG reaching goal or insulin dose exceeding > 0.5 units/kg/day, clinicians may sometimes continue up-titration of basal insulin contrary to the recommendations of the American Diabetes Association (ADA) [16, 17] rather than intensifying therapy by adding other glucose-lowering medications to the patient’s regimen. This concept, known as “over-basalization,” may expose patients to an unnecessary risk of hypoglycemia and weight gain, resulting in greater healthcare costs [18]. A post hoc analysis of three insulin glargine titration studies found that FPG reduction becomes proportionally smaller with increasing dose of basal insulin, leveling at approximately 0.5 units/kg/day; this may, therefore, be considered an approximate cutoff point at which alternative therapeutic options beyond continued basal insulin titration should be considered [19]. In accordance with these findings, a recent pooled analysis of 15 randomized controlled trials investigated basal insulin intensification in patients already on high insulin doses. These studies showed that although there are some patients with a low risk of hypoglycemia in whom basal insulin doses > 0.5 units/kg/day may be appropriate, overall continued up-titration to doses > 0.5 units/kg does not appear to improve glycemic control and is associated with increased weight gain and higher risk of hypoglycemia [20]. In the current study, participants receiving doses of < 0.5 units/kg/day insulin in the iGlarLixi arm showed a greater reduction in weight from baseline to study end than those on a higher insulin dose. No significant differences were observed in the incidence rates of hypoglycemia, GI AEs, and percentages of participants unable to achieve A1C < 7.0% (< 53 mmol/mol) between participants receiving either ≤ 0.5 unit/kg/day or > 0.5 unit/kg/day of insulin in both the iGlarLixi and iGlar arms.
As expected, participants treated with iGlarLixi reported a higher incidence of GI AEs compared with those who received iGlar. However, the incidence was similar in both iGlarLixi dose groups and lower than that reported for lixisenatide as a standalone therapy [21]. This supports previous findings that suggest that the gradual increase in the lixisenatide dose, which parallels the iGlar titration with iGlarLixi, mitigates GI AEs, including at higher doses [11]. Also, participants receiving the maximum dose of iGlar had significantly greater weight gain compared with those receiving lower doses of iGlar. Participants in the iGlarLixi arm experienced small decreases in weight, with no significant differences in weight change between those on the maximum dose or lower doses. This supports previous findings that suggest that the lixisenatide component of iGlarLixi mitigates the weight gain associated with iGlar even at the highest dose [22].
The results of our study are limited by the standard constraints associated with post hoc analyses of subgroups of data. The issue of statistical significance must be treated with caution since the analysis likely has insufficient power to detect such a difference between the subgroups. While these analyses do not replace data from specifically designed trials, they do provide a valuable insight into the patient characteristics associated with a higher likelihood of requiring the maximum basal insulin dose and the impact of maximum doses on patient outcomes.
Conclusions
In conclusion, the majority of participants in the LixiLan-L trial did not require treatment with the maximum dose of iGlarLixi (60 units/day) to achieve ADA-recommended glycemic targets. Our analysis indicates that there are key differences in the clinical phenotype of those patients who require maximum doses compared with those who do not. Patients more likely to require maximum doses of treatment were of younger age, with higher body weight, BMI, FPG, and insulin dose, all characteristics associated with greater insulin resistance. Even at the maximum doses employed in the study, iGlarLixi provided significantly greater glucose-lowering efficacy and modest weight benefit without increased risk of hypoglycemia for those participants compared with iGlar.
References
Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411–7.
Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18:401–9.
Calvert MJ, McManus RJ, Freemantle N. Management of type 2 diabetes with multiple oral hypoglycaemic agents or insulin in primary care: retrospective cohort study. Br J Gen Pract. 2007;57:455–60.
Nichols GA, Koo YH, Shah SN. Delay of insulin addition to oral combination therapy despite inadequate glycemic control: delay of insulin therapy. J Gen Intern Med. 2007;22:453–8.
DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95.
Balena R, Hensley IE, Miller S, Barnett AH. Combination therapy with GLP-1 receptor agonists and basal insulin: a systematic review of the literature. Diabetes Obes Metab. 2013;15:485–502.
Vora J. Combining incretin-based therapies with insulin: realizing the potential in type 2 diabetes. Diabetes Care. 2013;36(Suppl 2):S226–32.
Horowitz M, Rayner CK, Jones KL. Mechanisms and clinical efficacy of lixisenatide for the management of type 2 diabetes. Adv Ther. 2013;30:81–101.
Soliqua®. Prescribing information. http://products.sanofi.us/Soliqua100-33/Soliqua100-33.pdf. Accessed March 2019.
Suliqua®. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004243/WC500224673.pdf. Accessed June 2018.
Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39:1972–80.
Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39:2026–35.
McAdam-Marx C, Yu J, Bouchard J, Aagren M, Brixner DI. Comparison of daily insulin dose and other antidiabetic medications usage for type 2 diabetes patients treated with an analog basal insulin. Curr Med Res Opin. 2010;26:191–201.
Borah BJ, Darkow T, Bouchard J, Aagren M, Forma F, Alemayehu B. A comparison of insulin use, glycemic control, and health care costs with insulin detemir and insulin glargine in insulin-naive patients with type 2 diabetes. Clin Ther. 2009;31:623–31.
Umpierrez GE, Skolnik N, Dex T, Traylor L, Chao J, Shaefer C. When basal insulin is not enough: a dose-response relationship between insulin glargine 100 units/mL and glycaemic control. Diabetes Obes Metab. 2019. https://doi.org/10.1111/dom.13653 [Epub ahead of print].
American Diabetes Association. Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S1–193.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–701.
Shaefer C, Traylor L, Gao L, Dex T, Sepe P, Skolnik N. Exploratory study of a dose–response curve for basal insulin. Diabetes. 2015;64(Suppl 1): Abstract 253.
LaSalle JR, Berria R. Insulin therapy in type 2 diabetes mellitus: a practical approach for primary care physicians and other health care professionals. J Am Osteopath Assoc. 2013;113:152–62.
Reid T, Gao L, Gill J, et al. How much is too much? Outcomes in patients using high-dose insulin glargine. Int J Clin Pract. 2016;70:56–65.
Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care. 2013;36:2489–96.
Rosenstock J, Diamant M, Aroda VR, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan proof-of-concept randomized trial. Diabetes Care. 2016;39:1579–86.
Acknowledgments
Funding
This study was funded by Sanofi US, Inc., who also funded the journal’s Rapid Service Fee and Open Access fee. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
The authors received writing/editorial support in the preparation of this manuscript provided by Lisa Longato, PhD, Yasmin Issop, PhD, and Luke Shelton, PhD, of Excerpta Medica, funded by Sanofi US, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors contributed to study design, data analysis, and critical review of this manuscript.
Disclosures
Lawrence Blonde has acted as a consultant for AstraZeneca, Gilead Sciences Inc., Janssen Pharmaceuticals, Inc., Merck and Co., Inc., Novo Nordisk, and Sanofi; as a speaker for Janssen Pharmaceuticals, Inc., Novo Nordisk, and Sanofi; and has received research support from Janssen Pharmaceuticals, Inc., Lexicon Pharmaceuticals, Inc., Merck and Co., Inc., Novo Nordisk, and Sanofi. Timothy S. Bailey has acted as a consultant for Abbott, Capillary Biomedical, Eli Lilly, Medtronic, Novo Nordisk, and Sanofi; and has received speaker honoraria from Abbott, MannKind, Medtronic, Novo Nordisk, Sanofi, and Senseonics; and research support from Abbott, Ascensia, BD, Boehringer Ingelheim, Calibra Medical, Capillary Biomedical, Companion Medical, Dance Biopharm, Dexcom, Diasome, Eli Lilly, Glysens, Kowa, Lexicon, Medtronic, Novo Nordisk, POPS! Diabetes Care, Sanofi, Senseonics, Taidoc, vTv Therapeutics, Xeris, and Zealand. Jason Chao is an employee at Xinyi, Inc. and under contract with Sanofi US, Inc. Terry A. Dex is an employee of Sanofi US, Inc. Juan Pablo Frias is on the advisory panel of AstraZeneca and Sanofi; is a consultant for AstraZeneca, BMS, and Sanofi; has received research support from AbbVie, AstraZeneca, Boehringer Ingelheim, BMS, Eli Lilly, Ionis, Janssen Pharmaceuticals, Inc., Johnson and Johnson, Lexicon, Ligand, Merck & Co., Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi, Theracos, and vTv; and is on the speakers’ bureau of Sanofi. Luigi F. Meneghini is a consultant and advisory board member of Sanofi Aventis and Novo Nordisk. Michelle Roberts is an employee at Sanofi US, Inc. Vanita R. Aroda is a consultant for Adocia, AstraZeneca, BD, Novo Nordisk, Sanofi, and Zafgen; has received research support from Astra Zeneca/BMS, Calibra, Eisai, Janssen, Novo Nordisk, Sanofi, and Theracos; and her spouse is an employee at Merck Research Laboratories.
Compliance with Ethics Guidelines
As previously reported [11], the LixiLan-L trial was designed and monitored in accordance with Good Clinical Practice, the International Conference on Harmonization, and the Declaration of Helsinki. Institutional review boards or ethics committees at each study site approved the protocol. Each patient gave written informed consent.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8846222.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Blonde, L., Bailey, T.S., Chao, J. et al. Clinical Characteristics and Glycemic Outcomes of Patients with Type 2 Diabetes Requiring Maximum Dose Insulin Glargine/Lixisenatide Fixed-Ratio Combination or Insulin Glargine in the LixiLan-L Trial. Adv Ther 36, 2310–2326 (2019). https://doi.org/10.1007/s12325-019-01033-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01033-1