Abstract
Introduction
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist. In the SURPASS-AP-Combo trial, once-weekly tirzepatide was associated with improved glycemic control and weight loss versus insulin glargine and was generally well tolerated in an Asia-Pacific, predominately Chinese, population with type 2 diabetes (T2D). This post hoc subgroup analysis of SURPASS-AP-Combo assessed the potential influence of patient baseline characteristics on the efficacy and safety of tirzepatide.
Methods
Changes from baseline to week 40 in HbA1c, body weight, fasting serum glucose (FSG), and daily glucose average from self-measured blood glucose profiles were analyzed by potential influential factors including age (< 65, ≥ 65 years), sex, baseline HbA1c (≤ 8.5, > 8.5%), body mass index (BMI) (< 25, ≥ 25 kg/m2), body weight (< 75, ≥ 75 kg), duration of diabetes (< 10, ≥ 10 years), and concomitant oral antihyperglycemic medications (metformin, metformin plus sulphonylurea). Gastrointestinal adverse events and hypoglycemia were also evaluated.
Results
At week 40, all tirzepatide doses were associated with reduced HbA1c, body weight, FSG, and daily glucose average from baseline in all subgroups. Greater HbA1c reductions were achieved in patients with higher baseline HbA1c across all tirzepatide doses, higher body weight with 10 mg and younger age with 15 mg tirzepatide. Greater reductions in body weight were observed in patients with higher body weight across all tirzepatide doses, lower baseline HbA1c with 5 mg and higher BMI with 5 mg tirzepatide.
Conclusions
In this post hoc analysis, tirzepatide was associated with reduced blood glucose and body weight in a predominantly Chinese population with T2D across different subgroups, consistent with previous reports for tirzepatide.
Clinical Trial Registration
NCT04093752.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Type 2 diabetes represents a major clinical burden worldwide, including China |
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist that was shown to improve glycemic control and weight loss in Chinese patients with type 2 diabetes in the SURPASS-AP-Combo trial |
This subgroup analysis of the SURPASS-AP-Combo trial assessed the potential influence of baseline characteristics on the efficacy of tirzepatide including age, sex, baseline HbA1c, body mass index, body weight, duration of diabetes, and concomitant oral antihyperglycemic medications. Safety analyses included the incidence of gastrointestinal adverse events (AEs) and hypoglycemia |
What was learned from the study? |
Tirzepatide was associated with reduced blood glucose and body weight in the different subgroups, consistent with previous reports for tirzepatide |
These results support the use of tirzepatide in the Chinese population across different patient subgroups |
Introduction
In recent years, there has been epidemic growth in the number of individuals with diabetes worldwide, increasing from 422 million in 2014 to 529 million in 2021 [1, 2], primarily attributed to the escalating prevalence of type 2 diabetes (T2D) [3]. Concerningly, the global prevalence of diabetes is forecast to increase further in the coming years, by 25% by 2030 and 51% by 2045 [4]. Current data suggest that 60% of people with diabetes reside in Asia, with the highest prevalence in East Asia [5]. In 2021, the overall incidence of diabetes in China was estimated at > 140 million [6]. This rising prevalence of diabetes represents a major burden to patients, caregivers and healthcare systems worldwide [6]. Notably, the adult population affected by T2D is heterogenous, exhibiting diverse clinical characteristics and associated comorbidities. For example, East-Asian patients with T2D have a younger age of onset and lower body mass index (BMI) ranges compared with white patients [7].
Tirzepatide is a once-weekly, glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 (GIP/GLP-1) receptor agonist that has demonstrated clinically meaningful improvements in glycemic control and weight loss, along with a favorable safety profile in the global SURPASS 1–6 and Japanese SURPASS J-mono and J-Combo clinical trials, which included patients with T2D across various disease durations and treatment backgrounds [8,9,10,11,12,13,14,15]. Based on the findings from these clinical trials, tirzepatide was the first GIP/GLP-1 receptor agonist approved for treating T2D and is also approved for chronic weight management in the US [16]. Currently, tirzepatide is under investigation for the treatment of T2D and obesity in China.
SURPASS-AP-Combo was, to our knowledge, the first multicenter, phase 3 clinical trial to investigate the efficacy and safety of once-weekly tirzepatide versus insulin glargine in a predominantly Chinese population with T2D [17]. The results from this study were consistent with the findings of the global and Japanese SURPASS trials, demonstrating superior glycemic control and weight reduction with tirzepatide and also a safety profile in line with previous trials of tirzepatide [8,9,10,11,12,13,14,15].
To better understand the effects of once-weekly tirzepatide in an Asia-Pacific, predominately Chinese, population with T2D, we conducted a post hoc subgroup analysis of the SURPASS-AP-Combo to investigate the potential influence of baseline characteristics on the efficacy of once-weekly tirzepatide 5, 10 and 15 mg. Safety analyses included the incidence of gastrointestinal AEs and hypoglycemia.
Methods
Study Design
This was a post hoc analysis of SURPASS-AP-Combo, a randomized, open-label, multicenter, parallel-arm, phase 3 trial (ClinicalTrials.gov registration: NCT04093752). The study design and primary results have been published previously [17]. Briefly, the study recruited patients with T2D from 66 sites across China (n = 43), South Korea (n = 13), Australia (n = 6) and India (n = 4) and comprised a 1-week screening period, a 2-week lead-in period, a 40-week treatment period and a 4-week safety follow-up period. The study protocol was approved by Ethics Review Boards at each site, and written informed consent was collected from all participants. The study was conducted in accordance with the principles of international ethics guidelines, including the Declaration of Helsinki, and applicable local laws and regulations.
Patients and Treatment
The eligibility criteria for this trial have been published previously [17]; a summary of the inclusion and exclusion criteria can be found in the Supplementary Materials. Eligible patients were randomized 1:1:1:1 to receive once-weekly tirzepatide 5, 10, 15 mg or once-daily insulin glargine, stratified by baseline HbA1c (≤ 8.5, > 8.5%), country and use of concomitant oral antidiabetic treatments (metformin alone, metformin plus a sulfonylurea). Tirzepatide was initiated at 2.5 mg for all patients and increased by 2.5 mg every 4 weeks until the target dose was reached. All patients continued background therapy with metformin (± sulfonylurea) at the same pre-study dose. Rescue antihyperglycemic therapy was allowed for patients with severe, persistent hyperglycemia who met the pre-defined criteria.
Study Assessments and Subgroups
The current analyses evaluated efficacy data in patients included in SURPASS-AP-Combo who received tirzepatide (5, 10 and 15 mg). A patient flow chart is provided in Supplementary Fig. 1. Subgroups were defined according to the following key potential influential factors: age (< 65, ≥ 65 years), sex (male, female), baseline HbA1c (≤ 8.5, > 8.5%), baseline BMI (< 25, ≥ 25 kg/m2), baseline body weight (< 75, ≥ 75 kg), duration of diabetes (< 10, ≥ 10 years) and concomitant oral antidiabetic medication (OAM) use (metformin, metformin plus sulfonylurea). Baseline characteristics and the following efficacy endpoints at week 40 were evaluated across the predefined subgroups: change from baseline in HbA1c, body weight, fasting serum glucose (FSG) and daily glucose average calculated from 7-point self-measured blood glucose (SMBG) profiles. Safety analyses included the incidence of gastrointestinal adverse events (AEs) and hypoglycemia.
Statistical Analysis
Efficacy analyses of change from baseline to week 40 were conducted using a mixed model for repeated measures (MMRM) with treatment, subgroup, country, baseline OAM use (metformin, metformin plus sulfonylurea), baseline HbA1c category (≤ 8.5, > 8.5%), subgroup, time, subgroup-by-time interaction, treatment-by-time interaction, treatment-subgroup-time three-way interaction and baseline value as covariates. Least-square (LS) means, 95% confidence intervals (CIs) and P values for the comparisons between subgroups were computed from the MMRM model. For analyses of HbA1c, the baseline HbA1c category was not included in the model. The subgroups by baseline HbA1c (≤ 8.5, > 8.5%) and concomitant OAM use (metformin, metformin plus sulphonylurea) were only included once in the model. An unstructured covariance structure was used to model the relationship of intra-patient errors. Gastrointestinal AEs and hypoglycemia were reported as percentages, calculated based on the number of patients in each subgroup category.
Efficacy was evaluated using data obtained during the treatment period (from all randomized tirzepatide treated patients who received ≥ 1 dose of study drug) and excluding data after initiating rescue antihyperglycemic medication or discontinuation of study drug. Safety analyses used all data collected from the start of treatment to the end of the safety follow-up period. All tests of treatment effect were conducted at a two-sided alpha level of 0.05. All analyses were performed using SAS Version 9.4.
Results
Baseline Characteristics
A total of 687 patients were included for this subgroup analysis, who received tirzepatide 5 mg (n = 230), tirzepatide 10 mg (n = 228) or tirzepatide 15 mg (n = 229). A greater proportion of patients were male versus female across the tirzepatide treatment subgroups (5, 10 and 15 mg) (Table 1). The mean (SD) overall age of patients across the tirzepatide treatment groups ranged from 53.1 (11.2) to 54.3 (11.6) years. The mean (SD) BMI of patients across the tirzepatide treatment groups ranged from 27.7 (3.8) to 28.1 (3.9) kg/m2. Overall, baseline characteristics were generally well balanced across the tirzepatide treatment groups (Table 1).
Efficacy
Changes from Baseline in HbA1c
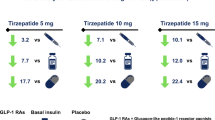
All doses of tirzepatide (5, 10 and 15 mg) resulted in a reduction in HbA1c from baseline to week 40 across each subgroup, irrespective of age, sex, baseline HbA1c, BMI, body weight, duration of diabetes and concomitant OAM use (Fig. 1). For the individual analyses by subgroup, LS mean (standard error [SE]) reductions in HbA1c from baseline to 40 weeks were significantly greater in patients with a higher baseline HbA1c (≤ 8.5 vs. > 8.5%) with all tirzepatide doses: tirzepatide 5 mg – 2.52% (0.093) vs. – 1.76% (0.105) P < 0.001; tirzepatide 10 mg – 2.95% (0.099) vs. – 1.78% (0.103), P < 0.001; tirzepatide 15 mg – 3.01% (0.100) vs. – 1.86% (0.102), P < 0.001 (Fig. 1). LS mean (SE) reductions in HbA1c were significantly greater in younger patients (< 65 vs. ≥ 65 years) with tirzepatide 15 mg: – 2.53% (0.087) vs. – 2.08% (0.160), respectively, P = 0.011 (Fig. 1). Furthermore, a numerically greater LS mean (SE) reduction in HbA1c was observed in patients with a higher baseline body weight (≥ 75 vs. < 75 kg) with tirzepatide 10 and 15 mg, and the difference reached statistical significance for tirzepatide 10 mg: – 2.56% (0.111) vs. – 2.26% (0.105), respectively, P = 0.040 (Fig. 1). Comparatively small and non-statistically significant differences in the reduction in HbA1c from baseline to week 40 were observed between the remaining subgroups across the tirzepatide treatment groups (P ≥ 0.05).
Changes from baseline to week 40 in HbA1c (%) stratified by potential influential factors. P-value from MMRM model. BMI body mass index; HbA1c hemoglobin A1c; LS mean least-square mean; MMRM mixed-model for repeated measures; n number of patients who were randomized and received at least one dose of study drug; OAM oral antihyperglycemic medication; TZP tirzepatide
Change from Baseline in Body Weight
Across all subgroups, tirzepatide was associated with a reduction in mean body weight from baseline to week 40, irrespective of age, sex, baseline HbA1c, BMI, body weight, duration of diabetes and use of concomitant OAMs (Fig. 2). LS mean (SE) reductions in body weight from baseline to 40 weeks were significantly greater in patients with a higher baseline body weight (≥ 75 vs. < 75 kg) across all tirzepatide doses: tirzepatide 5 mg – 5.83 kg (0.460) vs. – 4.13 kg (0.493), respectively, P = 0.010; tirzepatide 10 mg – 7.89 kg (0.511) vs. – 6.34 kg (0.473), respectively, P = 0.022; tirzepatide 15 mg – 8.09 kg (0.491) vs. – 6.31 kg (0.485), respectively, P = 0.008 (Fig. 2). Similarly, patients with a higher baseline BMI (≥ 25 vs. < 25 kg/m2) receiving tirzepatide 10 and 15 mg also experienced a numerically greater LS mean (SE) reduction in body weight, and this was statistically significant in the 5 mg treatment group: – 5.42 kg (0.385) vs. – 3.66 kg (0.714), P = 0.026 (Fig. 2). A lower baseline HbA1c (≤ 8.5% vs. > 8.5%) was associated with a significantly greater LS mean (SE) reduction in body weight from baseline to week 40 with tirzepatide 5 mg: – 5.85 kg (0.509) vs. – 4.41 kg (0.447), respectively, P = 0.029 (Fig. 2). In addition, LS mean (SE) reductions in body weight were numerically greater in younger patients (< 65 vs. ≥ 65 years) across all tirzepatide treatment groups, and the difference reached significance for patients receiving tirzepatide 15 mg: – 7.90 kg (0.393) vs. – 4.67 kg (0.728), respectively, P < 0.001 (Fig. 2). No statistically significant differences in reduction in body weight were observed in the remaining subgroups across the tirzepatide treatment groups (P ≥ 0.05).
Changes from baseline to week 40 in body weight (kg) stratified by potential influential factors. P-value from MMRM. BMI body mass index; HbA1c hemoglobin A1c; LS mean least-square mean; MMRM mixed-model for repeated measures; n number of patients who were randomized and received at least one dose of study drug; OAM oral antihyperglycemic medication; TZP tirzepatide
Change from Baseline in FSG
Tirzepatide (5, 10 and 15 mg) was associated with a reduction in mean FSG from baseline to week 40, irrespective of age, sex, baseline HbA1c, BMI, body weight, duration of diabetes or use of concomitant OAMs (Fig. 3). A greater LS mean (SE) reduction in FSG from baseline to week 40 was observed in patients with a lower baseline HbA1c (≤ 8.5% vs. > 8.5%) receiving tirzepatide 5 mg: – 63.16 mg/dl (3.678) vs. – 53.82 mg/dl (3.376), respectively, P = 0.033 (Fig. 3). Similar reductions in FSG from baseline to week 40 were observed in the remaining subgroups, across the tirzepatide treatment groups (P ≥ 0.05) (Fig. 3).
Changes from baseline to week 40 in FSG (mmol/l) stratified by potential influential factors. P-value from MMRM. BMI body mass index; FSG fasting serum glucose; HbA1c hemoglobin A1c; LS mean least-square mean; MMRM mixed-model for repeated measures; n number of patients who were randomized and received at least one dose of study drug; OAM oral antihyperglycemic medication; TZP tirzepatide
Reduction in Daily Glucose Average of SMBG Profiles
At week 40, all doses of tirzepatide were associated with reductions from baseline in the daily glucose average from SMBG profiles, irrespective of age, sex, baseline HbA1c, BMI, body weight, duration of diabetes and use of concomitant OAMs (Supplementary Fig. 2). A significantly greater LS mean (SE) reduction in daily glucose average from SMBG profiles was observed in younger patients (< 65 vs. ≥ 65 years) receiving tirzepatide 15 mg: – 5.04 mmol/l (0.180) vs. –4.18 mmol/l (0.288), respectively, P = 0.003 (Supplementary Fig. 2). Among patients receiving tirzepatide 5 mg, a lower baseline HbA1c (≤ 8.5% vs. > 8.5%) was associated with a significantly greater LS mean (SE) reduction in daily glucose average from SMBG profile: – 4.75 mmol/l (0.214) vs. – 4.15 mmol/l (0.198), respectively, P = 0.014 (Supplementary Fig. 2). Conversely, in the tirzepatide 10 and 15 mg treatment groups, a numerically greater reduction in SMBG profiles was observed in patients with a higher baseline HbA1c, but the difference did not reach statistical significance (Supplementary Fig. 2). In the remaining subgroups, similar reductions in SMBG profiles from baseline to week 40 were observed across the tirzepatide treatment groups (P ≥ 0.05) (Supplementary Fig. 2).
Safety
Gastrointestinal AEs and Hypoglycemia Incidence
The proportion of patients reporting treatment-emergent gastrointestinal AEs was generally similar across each subgroup (Table 2). Across all tirzepatide doses, the total incidence of hypoglycemia was broadly comparable among the patient subgroups (Table 3). Patients with a BMI of < 25 vs. ≥ 25 kg/m2 and a body weight of < 75 vs. ≥ 75 kg experienced a higher incidence of hypoglycemia with tirzepatide 10 mg (Table 3). Notably, across all tirzepatide doses, patients using metformin plus sulfonylurea versus metformin alone experienced a higher incidence of hypoglycemia (Table 3).
Discussion
This post hoc subgroup analysis of SURPASS-AP-Combo is the first to our knowledge to evaluate the efficacy of once-weekly tirzepatide stratified by baseline characteristics in an Asian-Pacific, predominantly Chinese, population. All three doses of tirzepatide were associated with reductions in mean HbA1c, body weight, FSG and daily glucose average from SMBG profiles across all investigated subgroups. These findings are consistent with the primary results of SURPASS-AP Combo [17]. Positive associations were observed between several patient subgroups and mean reduction in HbA1c, body weight, FSG and daily glucose average from SMBG profiles from baseline to week 40, which may highlight potential patient populations that may benefit from tirzepatide treatment.
In this analysis, reductions in mean HbA1c from baseline to week 40 were most influenced by patient HbA1c levels at baseline; patients with a higher baseline HbA1c experienced a significantly greater reduction in HbA1c across all tirzepatide doses. A similar observation was reported in a post hoc analysis of the global phase 3 SURPASS clinical trial program that evaluated glycemic control by baseline HbA1c (≤ 8.5% or > 8.5%) [18]. In the present study, a numerically greater reduction in HbA1c from baseline to week 40 was achieved by patients with a higher baseline body weight receiving tirzepatide 10 and 15 mg, and the difference reached statistical significance in patients receiving tirzepatide 10 mg. This finding may suggest an added value of weight loss on glucose reduction in patients with T2D and overweight or obesity. In this study, a significantly greater reduction in HbA1c and SMBG profile was observed in younger patients receiving tirzepatide 15 mg. Interestingly, a previous study in patients with uncontrolled T2D on insulin showed that the glucose-lowering effect of liraglutide was dependent on beta cell function [19, 20]. However, we observed that blood glucose control, including reductions in HbA1c, FSG and SMBG profiles, was similar across the three tirzepatide treatment groups by duration of T2D (< 10 vs. ≥ 10 years), which is consistent with the results of a previous subgroup analysis from the global phase 3 SURPASS studies that investigated the glycemic effect of tirzepatide by duration of diabetes [21]. Additional analysis is warranted to investigate the correlation between the glucose-lowering effect, patient age and the duration of diabetes as well as beta-cell function.
Our results show that all doses of tirzepatide were associated with improvements in body weight, FSG and daily glucose average from SMBG profiles across the patient subgroups at week 40. Notably, reductions in body weight were significantly greater in patients with a higher baseline body weight across all doses of tirzepatide and in the tirzepatide 5 mg group for patients with a higher baseline BMI. These findings were consistent with a post hoc analysis of SURPASS-1 through -5, which showed that absolute weight change was generally greater among patients with higher BMI categories [22]. In the present study, a statistically significant reduction in body weight was observed in patients with a lower baseline HbA1c in the tirzepatide 5 mg group, consistent with results observed with GLP-1 RAs [23, 24]. In the present analysis, the reduction in body weight from baseline was significantly greater in younger patients (< 65 years) receiving tirzepatide 15 mg. However, this result was not consistently observed across the tirzepatide treatment groups. In the baseline HbA1c subgroup (≤ 8.5% vs. > 8.5%), a statistically significant greater reduction in FSG and SMBG profiles was observed in patients with lower baseline HbA1c with tirzepatide 5 mg.
The overall incidence of treatment-emergent gastrointestinal AEs and hypoglycemia at week 40 were generally similar across the different patient subgroups, with some variability observed in specific subgroups. For example, across all tizepatide treatment groups, a higher incidence of hypoglycemia was observed in patients using metformin plus sulphonylurea compared to metformin alone, which is consistent with previous studies [9, 17]. In the present study, baseline BMI (< 25 kg/m2) and body weight (< 75 kg) were associated with a significantly greater incidence of hypoglycemia with tirzepatide 10 mg. Interestingly, in a previous East Asian focused study (predominantly Japanese patients) that investigated the safety and efficacy of tirzeatide according to age and baseline BMI, the rate of AE-related discontinuations was highest within the BMI < 25 kg/m2 and > 65 years of age subgroup across all tirzepatide doses [25]. In our analysis, among patients receiving tirzepatide 5 mg, the incidence of total hypoglycemia was significantly higher in patients with a baseline HbA1c ≤ 8.5 vs. > 8.5%. This finding is similar to previous observations in patients receiving a GLP-1 receptor agonist [23]. In addition, our study found that older patients (> 65 years) experienced a significantly greater total incidence of gastrointestinal AEs with tirzepatide 10 mg. However, in the previously mentioned East Asian study of tirzepatide stratified by age and BMI subgroups, no patterns between gastrointestinal AEs and age were reported [25].
The limitations of this study include those inherent to a post hoc subgroup analysis, such as the uneven disribution of patients within the age and BMI baseline subgroups, with comparably more patients < 65 years old and with a BMI ≥ 25 kg/m2. In addition, as this was a post hoc analysis, the statistical and clinical significance of nominal P values should be interpretted with caution.
Conclusion
In summary, the results of this post hoc subgroup analysis demonstrated improvements in glycemic control, body weight and daily glucose average from SMBG profiles across all tirzepatide doses (5, 10, and 15 mg) regardless of patient age, sex, baseline HbA1c, BMI, body weight, or duration of diabetes or use of concomitant OAMs in a predominantly Chinese population with T2D.
Data Availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019. Results. Institute for Health Metrics and Evaluation. 2020 (https://vizhub.healthdata.org/gbd-results/). Accessed Jan 2024.
GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–34.
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al KJ. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–11.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157: 107843.
Ramachandran A, Snehalatha C, Shetty AS, Nanditha A. Trends in prevalence of diabetes in Asian countries. World J Diabetes. 2012;3(6):110–7.
International Diabetes Federation. IDF Diabetes Atlas teB, Belgium: 2021. [Available from: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf. Accessed Jan 2024.
Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care. 2011;34(8):1741–8.
Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. 2022;327(6):534–45.
Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398(10313):1811–24.
Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–15.
Ludvik B, Giorgino F, Jódar E, Frias JP, Fernández Landó L, Brown K, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583–98.
Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–55.
Inagaki N, Takeuchi M, Oura T, Imaoka T, Seino Y. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10(9):623–33.
Kadowaki T, Chin R, Ozeki A, Imaoka T, Ogawa Y. Safety and efficacy of tirzepatide as an add-on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (SURPASS J-combo): a multicentre, randomised, open-label, parallel-group, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10(9):634–44.
Rosenstock J, Frías JP, Rodbard HW, Tofé S, Sears E, Huh R, et al. Tirzepatide vs insulin lispro added to basal insulin in type 2 diabetes: the SURPASS-6 randomized clinical trial. JAMA. 2023;330(17):1631–40.
FDA Approves New Medication for Chronic Weight Management. 2023 [Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management#:~:text=888-INFO-FDA%20The%20FDA%20approved%20Zepbound%20%28tirzepatide%29%20injection%20for,a%20reduced%20calorie%20diet%20and%20increased%20physical%20activity. Accessed Jan 2024.
Gao L, Lee BW, Chawla M, Kim J, Huo L, Du L, et al. Tirzepatide versus insulin glargine as second-line or third-line therapy in type 2 diabetes in the Asia-Pacific region: the SURPASS-AP-Combo trial. Nat Med. 2023;29(6):1500–10.
Aleppo G, De Block C, Levine JA, Gomez-Valderas E, Benneyworth BD. 717-P: glycemic control with tirzepatide in people with type 2 diabetes by baseline HbA1c = 8.5% or > 8.5%. Diabetes. 2022. https://doi.org/10.2337/db22-717-P
Wilbrink FJ, Mudde AH, Mulder AH, Bhansing KJ. Disease duration as an indicator of the efficacy of liraglutide in patients with type 2 diabetes mellitus. J Diabetes Investig. 2018;9(4):979–80.
Usui R, Sakuramachi Y, Seino Y, Murotani K, Kuwata H, Tatsuoka H, et al. Retrospective analysis of liraglutide and basal insulin combination therapy in Japanese type 2 diabetes patients: the association between remaining β-cell function and the achievement of the glycated hemoglobin target 1 year after initiation. J Diabetes Investig. 2018;9(4):822–30.
De Block C, Mathieu C, Sapin H, Kiljanski JI, Peleshok J. 727-P: glycemic effect of tirzepatide by duration of diabetes. Diabetes. 2022. https://doi.org/10.2337/db22-727-P.
Kwan A, Maldonado JM, Wang H, Rasouli N, Wilding J. 719-P: tirzepatide induces weight loss in patients with type 2 diabetes regardless of baseline BMI: a post hoc analysis of SURPASS-1 through -5 studies. Diabetes. 2022. https://doi.org/10.2337/db22-719-P.
Gallwitz B, Dagogo-Jack S, Thieu V, Garcia-Perez LE, Pavo I, Yu M, et al. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes Metab. 2018;20(2):409–18.
Pantalone KM, Patel H, Yu M, Landó LF. Dulaglutide 1.5 mg as an add-on option for patients uncontrolled on insulin: subgroup analysis by age, duration of diabetes and baseline glycated haemoglobin concentration. Diabetes Obes Metab. 2018;20(6):1461–9.
Kiyosue A, Dunn JP, Cui X, Hickey A, Hirase T, Imaoka T, et al. Safety and efficacy analyses across age and body mass index subgroups in East Asian participants with type 2 diabetes in the phase 3 tirzepatide studies (SURPASS programme). Diabetes Obes Metab. 2023;25(4):1056–67.
Acknowledgements
We thank the participants of this study.
Medical Writing/Editorial Assistance
Editorial support was provided by Louise Hulme, MSc, from Rude Health Consulting Limited.
Funding
The study, editorial assistance, and journal Rapid Service Fee were funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
Linong Ji, Yan Bi and Song Lu contributed to the acquisition and interpretation of data. Jiani Tang contributed to the conception of study, and Liying Du contributed to the analysis and interpretation of data. All authors contributed to revising and approving the final version for submission.
Corresponding author
Ethics declarations
Conflict of interest
Linong Ji reports having received consulting or lecture fees from Eli Lilly and Company, Novo Nordisk, Merck, Bayer, Sanofi-Aventis, Roche, MSD, Metronics AstraZeneca, Boehinger Ingelheim, Abbott, Haisco Pharmaceutical. Yan Bi and Song Lu have nothing to disclose. Jiani Tang and Liying Du are employees of Eli Lilly and Company, and hold equity in Eli Lilly and Company.
Ethical Approval
The study protocol was approved by Ethics Review Boards at each site and written informed consent was collected from all participants. The study was conducted in accordance with the principles of international ethics guidelines, including the Declaration of Helsinki, and applicable local laws and regulations.
Additional information
Prior Publication: A poster from this study has been previously presented at The 25th Scientific Meeting of the Chinese Diabetes Society.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bi, Y., Lu, S., Tang, J. et al. Efficacy and Safety of Tirzepatide in Patients with Type 2 Diabetes: Analysis of SURPASS-AP-Combo by Different Subgroups. Diabetes Ther 15, 1125–1137 (2024). https://doi.org/10.1007/s13300-024-01561-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01561-2