Abstract
Bioethanol production by white rot fungus (Trametes versicolor), identified from fungal mixture in naturally decomposing wood samples, from hexoses and xylose was characterized. Results showed that T. versicolor can grow in culture, under hypoxic conditions, with various mixtures of hexoses and xylose and only xylose. Xylose was efficiently fermented to ethanol in media containing mixtures of hexoses and xylose, such as MBMC and G11XY11 media (Table 1), yielding ethanol concentrations of 20.0 and 9.02 g/l, respectively, after 354 h of hypoxic culture. Very strong correlations were found between ethanolic fermentation (alcohol dehydrogenase activity and ethanol production), sugar consumption and xylose catabolism (xylose reductase, xylitol dehydrogenase and xylulokinase activities) after 354 h in culture in MBMC medium. In a medium (G11XY11) containing a 1:1 glucose/xylose ratio, fermentation efficiency of total sugars into ethanol was 80% after 354 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Energy crops and lignocellulosic residues (such as woods and agricultural residues, which contain abundant potentially fermentable carbohydrates in the form of hexose and pentose polymers) have a huge potential as alternative, renewable bio-energy sources for major impending energy crisis in the world [1]. However, economically efficient conversion of such substrates into liquid bio-fuel, ethanol, remains challenging. Inter alia, for efficient fermentation of lignocellulosic residues to ethanol, cost-effective methods for fermenting d-xylose need to be developed, since it is the second most abundant fermentable carbohydrate in these residues [2].
Biological methods for using lignocellulosic biomass in ethanolic fermentation are becoming cost-effective. However, a major problem in industrial bio-ethanolic refineries is that the most commonly used microorganism, Saccharomyces cerevisiae, can only ferment certain mono- and disaccharides (such as glucose, fructose, maltose and sucrose) efficiently into ethanol. It cannot convert pentoses, which are also major components of lignocellulosic biomass [1]. Thus, it would be more cost-effective to use microorganisms that can convert both pentoses and hexoses into ethanol. The fungus Phanerochaete chrysosporium can reportedly convert pentoses and hexoses in lignocellulosic biomass into ethanol [3], and the yeasts Pichia stipitis, Candida shehatae and Pachysolen tannophilus can assimilate pentoses into ethanol, but only at rates fivefold lower than that of S. cerevisiae glucose fermentation [1]. In addition, S. cerevisiae has been successfully genetically engineered to metabolise pentoses (xylose and arabinose), but at insufficient rates for use in industrial bio-refineries; much lower than its glucose-fermentation rate [2].
The pentose sugars, arabinose and xylose, are converted to xylulose-5-phosphate before entering central carbon catabolism. Xylose is first reduced by xylose reductase (XR) to xylitol, which is then oxidised to xylulose by xylitol dehydrogenase (XDH). Xylulose is finally phosphorylated to xylulose-5-phosphate by (XK) xylulokinase [4]. Since low-oxygen conditions are required for efficient fermentation, mitochondrial respiration negatively affects it and in fermentation the NADH produced by the glycolytic pathway is re-oxidized through the fermentative pathway, producing the main derivatives, such as lactate and ethanol [5]. Ethanol is produced as a consequence of the decarboxylation of pyruvate, yielding acetaldehyde, and finally catalysis by alcohol dehydrogenase (ADH) [5].
In the present study, white rot fungus, Trametes versicolor, identified from fungal mixture in naturally decomposing wood samples in our laboratory [6], was further characterized. The objectives were to determine the ability of T. versicolor to produce ethanol from hexose and xylose mixtures, particularly a medium (MBMC; Table 1) containing sugars found in industrial spent sulphite liquor from Domsjö paper pulp factory Sweden, and the efficiency of the process. To this end, we compared biomass production, ethanol production, sugar utilization efficiencies and relevant enzyme activities (ADH, XR, XDH and XK) of T. versicolor cultures in media with various mixtures of hexose and xylose, and xylose-only substrates, under hypoxic and anoxic conditions.
Materials and Methods
Organisms, Media and Culture Conditions
T. versicolor (CBS 109428) culture was obtained from the CBS culture collection (The Netherlands) and grown in 300-ml bottles containing 100 ml of MBMC medium [7] including inorganic salts 7.5 g/l (NH4)2SO4, 3.0 g/l KH2PO4, 0.5 g/l MgSO4·7 H2O, 6.7 ml of trace metal solution and 0.7 ml of vitamin solution, 2.5 g/l yeast extract, 27 g/l mannose and 9.7 g/l glucose. Stock cultures were maintained for 3 weeks in the dark with shaking (150 rev min−1) at 27°C. Fungal cells 3 g/l fresh weight (FW) from stock culture was transferred to a MBMC medium including only glycerol (1.2 g/l) as carbon source for 7 days before each sugar experiment.
Growth Experiments
Portions containing 3 g/l (FW) of stock fungal culture (see above) were pelleted and added to 8-ml portions of media containing sugars in various ratios (Table 1) in 50-ml glass bottles, sealed with a rubber septum (SubaSeal, William Freeman Ltd, South Yorkshire, UK). Cultures were incubated directly as hypoxic culture conditions and anoxic culture conditions was maintained by flushing argon through inlet and outlet needles used to remove oxygen for 5 min simultaneously. The culture bottles containing the fungus were incubated with shaking (150 rev min−1) at 27°C. The FW was measured, and hyphae and media were analysed biochemically, in three random, 8-ml replicates sampled after 18, 66, 114, 234 and 354 h of incubation.
Genotype Verification
Hyphae were harvested from 15-day-old mother cultures of Trametes sp., isolated from an ethanol-producing fungal-mix maintained in our laboratory [6]. DNA was extracted from 100 mg of tissue using a DNA extraction mini-kit from Viogene (Sunnyvale, CA). Polymerase chain reaction (PCR) were performed in reaction mixtures (100 μl) containing 2 μl DNA (50–100 ng) solution, 10 μl ×10 PCR buffer, 1 μM ITS1-F forward primer [8] and ITS4 reverse primer [9], 1 mm deoxynucleotide triphosphate and 0.5 units of ampliTaq® DNA polymerase (Applied Biosystems). The amplified fragments were purified using a QIAquick PCR purification kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s protocol. Purified fragments were then sequenced via reactions with 200 fmol template and 10 pmol of the ITS1-F primer in 28 cycles of 95°C for 20 s, 50°C for 15 s and 60°C for 1 min in an ABI 377 sequencing apparatus (Applied Biosystems, Foster City, CA, USA). Sequences obtained were compared with those available in the NCBI database [10]. Sequences of primer pairs and construct sequences are listed in Supplementary Table 1.
Sugar Assays
Sets of three random replicates (8 ml each) of the MBMC and XY11 growth media were collected after 18, 66, 114, 234 and 354 h of cultivation to determine their xylose, mannose and glucose contents. Neutral sugars were quantified after conversion to alditol acetates and subsequent analysis by gas chromatography, as previously described [11], using myo-inositol as an internal standard. The alditol acetates were analysed using an Agilent 7890A GC system (Agilent Technologies, Santa Clara, CA) equipped with a DB-225 capillary column (15 m, 0.25 mm and 0.25 μm film thickness; J&W Scientific Inc., Folsom, CA) as stationary phase and a temperature programme beginning with 80°C for 2 min, followed by linear gradients from 80°C to 170°C at 30°C/min and from 170°C to 240°C at 4°C/min. A flame ionization detector was used to quantify eluting analytes.
Ethanol Assay
The ethanol contents of the growth media with different sugar combinations (Table 1) from incubations under anoxic and hypoxic conditions were quantified in three random replicates after, 18, 66, 114, 234 and 354 h, using gas chromatography, as previously described [6], and ethanol (1% to 10% dilutions) as a standard.
Enzyme Activity Assays
Sets of three random replicates (8 ml) of fungal hyphae grown in media containing different sugar combinations (Table 1) were used to determine their XR, XDH, XK and ADH activities after 18, 66, 114, 234 and 354 h of incubation, as follows. Crude protein was extracted from 50 mg of homogenised tissue by adding 400 μl of ice-cold disintegration buffer (pH 7) containing 0.1 M triethanolamine, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol and 0.5 mM EDTA. The extracts were then centrifuged at 16,000×g (4°C for 15 min). Enzyme activities of the resulting supernatants were determined according to [12]. Readings of 96-well micro-plates, with 10 μl extract and 190 μl reading buffer per well (and four standards per plate), were taken at A340 (25°C for 20 min), using a SPECTRA max ®190 spectrophotometer [13]. V blank and V max assays were performed for each sample in three replicates. Samples were randomly distributed to avoid local artefacts. The acquired data were analysed with SOFTmax ® PRO software using path length analysis. The enzyme activities were determined by adding samples to reaction mixtures containing (1) 100 mM triethanolamine (pH 7) and 0.2 mM NADPH (for XR), (2) 100 mM glycine (pH 9), 50 mM MgCl2 and 3 mM NAD+ (for XDH), (3) 50 mM Tris–HCl (pH 7.5), 2 mM MgCl2, 0.2 mM NADH, 8.5 mM xylulose, 0.2 mM phospoenol pyruvate, 10 U pyruvate kinase and 10 U lactate dehydrogenase (for XK), and (4) 100 mM glycine (pH 9), 5 mM NAD+ (for ADH), then initiating the reactions by adding 23 μl of 350 mM xylose, 20 μl of 300 mM xylitol, 1 μl of 2 mM ATP and 20 μl of 1.7 M ethanol, respectively.
Protein Determination
Protein concentrations of extracts of fungal hyphae were determined according to Bradford [14] with bovine serum albumin as a standard. The A595 of a 5-μl sample mixed with 195 μl of assay reagent was measured in 96-well micro-plates using SPECTRA max ®190 molecular device and SOFTmax ® PRO software.
Results and Discussion
T. versicolor was previously identified from decomposing wood samples obtained from the field [6], and in the present study, its genotype was re-confirmed by PCR analysis, using ITS1-F and ITS4 primers. Sequenced PCR products (Supplementary Table 1) were aligned with available sequences in the NCBI database [10] and were found to have up to 99% identity with T. versicolor (FJ810146.1). Therefore, T. versicolor (CBS 109428) from the CBS Culture Collection (The Netherlands) was used in all the experiments described in this paper.
T. versicolor Thrives in Media Containing Hexoses and Xylose Under Hypoxic Conditions
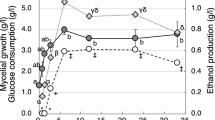
Using their extra-cellular enzymatic complexes basidiomycetous white rot fungi, such as T. versicolor, are responsible for extensive biodegradation of lignin in nature [15, 16]. The most rapid and extensive degradation caused by certain fungi, particularly white rot fungi, occurs in highly aerobic environments [15]. In the growth experiments presented here, with differing sugar combinations, T. versicolor grew exponentially, producing biomasses of 12.6 g/l FW and 7.2 g/l FW after 66 h in MBMC and XY11 media (Table 1), respectively, under hypoxic conditions (Fig. 1b). Furthermore, T. versicolor growth was stable in both media for up to 354 h (Fig. 1b). However, under anoxic conditions T. versicolor did not grow during the 354 h incubation period in either medium, in accordance with results of a previous study in which liquid culture bottle headspaces of T. versicolor cultures were flushed daily with 95% replacement of the atmospheric gases to promote typical growth [17].
In addition, growth behaviour in both media showed a typical pattern, the total biomass produced during the 354 h under hypoxic conditions being significantly higher in MBMC than in XY11 medium (Fig. 1b). The ability of T. versicolor to grow rapidly using various carbohydrate sources, including hexoses (glucose and mannose) and pentoses (xylose) explains its ability to thrive in diverse ecosystems. Moreover T. versicolor produces generative filamentous hyphae (Fig. 1a), like most Trametes species, with three types of structure: skeletal, generative and binding [18, 19], making it easy to handle. The ease of handling, stability, versatility and rapid multiplication of T. versicolor offer substantial advantages for its use in industrial bio-refineries [20]. Hence, there is intensive research into their use not only in bio-fuel generation, but also in bioremediation, effluent treatment, biosensors, synthetic chemistry and the pulp and paper, food, cosmetic and textile industries.
P. tannophilus shows enhanced aerobic growth and fermentation of xylose into ethanol after periodic additions of glucose, while similar additions to anaerobic cultures have no effect on its xylose utilization [21]. To determine the effects of varying combinations of glucose and xylose on the growth of T. versicolor, it was cultivated on media with various ratios of sugars (G11XY11, G6XY11 and G2XY11, containing 1:1, 1:1.8 and 1:5.5 glucose/xylose ratios, respectively; Table 1) in both hypoxic and anoxic conditions. The cultures were sampled after 18, 66, 114, 234 and 354 h at 27°C. Biomass (FW) production measurements indicated that growth was rapid for up to 66 h in all three glucose/xylose media (Fig. 3a) and was stable for up to 354 h under hypoxic conditions. However, under anoxic conditions no biomass production was detected. Furthermore, under hypoxic growth conditions, slight differences were detected in the biomass produced over 354 h between the three media (Fig. 3a). Our results support those of previous findings that efficient aeration of Rhizopus oryzae liquid cultures is important for rapid assimilation of hexoses and pentoses [7].
Sugar Utilization
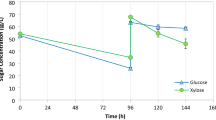
Studies of genetically engineered xylose-fermenting yeast has earlier shown that glucose utilization proceeds both promotion and inhibition of xylose usage [22] in mixed sugar fermentation, while aeration doubled the specific glucose-fermentation rate in P. tannophilus cultures [21]. To elucidate the sugar utilizing ability of T. versicolor in hexose and xylose mixtures, the sugars present in MBMC and XY11 media after 18, 66, 114, 234 and 354 h of cultivation at 27°C under hypoxic conditions were analysed. The results clearly showed that T. versicolor could utilize mannose, glucose and xylose simultaneously under hypoxic conditions in MBMC medium for up to 354 h (Fig. 2). This is a unique physiological characteristic of T. versicolor compared with other xylose-utilizing microorganisms, most of which use hexoses at higher rates than pentoses such as xylose in early growth stages [23, 24], while in our experiments T. versicolor utilized mannose, glucose and xylose simultaneously, consuming 32%, 44% and 36% of these sugars, respectively, within 66 h (Fig. 2). In addition, our data indicate that T. versicolor can effectively use xylose as a sole carbohydrate source over 354 h (Fig. 5a), after which ca. 90% of xylose had been utilized. Moreover, T. versicolor utilized 53% of the xylose during the first 18 h of culture (Fig. 5a). This further explains the physiological adaptability that T. versicolor has evolved, from its exposure to the diverse substrates in its environment.
In this study, among the hexoses T. versicolor showed a preference for glucose over mannose with concomitant consumption of xylose during the first 66 h of cultivation (Fig. 2). While, during the later growth period (66–354 h), 58%, 55% and 44% of the mannose, glucose and xylose were used, respectively (Fig. 2). Therefore, our sugar utilization analysis indicates that there was no hexose (glucose or mannose) co-substrate inhibition of xylose use, in contrast to an earlier report on genetically engineered xylose-fermenting S. cerevisiae [22]. S. cerevisiae takes up xylose by diffusion trough non-specific hexose transporters which have lower affinity for xylose than for glucose [2]. Therefore, xylose transport of S. cerevisiae is competitively inhibited by glucose, and xylose is consumed only after depletion of glucose [2]. However, our results strongly indicate that T. versicolor takes up both hexose (glucose and mannose) and pentose (xylose) simultaneously, while xylose is transported by the same system as hexose (Fig. 2). The importance of sugar transporter systems was demonstrated by Hamacher et al. [25], who showed that deletion of 18 Hxts transporter genes in a genetically modified S. cerevisiae strain (TMB 3201) capable of growth on xylose-based medium caused loss of the yeast’s ability to take up and grow on xylose. In addition, since no hexose co-substrate inhibition was observed, T. versicolor presumably has mechanisms allowing sugar catabolism under hypoxic conditions, including the continuous of supply reactants required for efficient glycolysis and ethanol fermentation (inter alia glucose, which elevates the intracellular metabolic pool of glyceraldehyde-3-phosphate, thus facilitating efficient operation of the pentose phosphate pathway; [21]).
In most fungi and xylose-fermenting yeasts (e.g. P. stipitis, P. tannophilus and C. shehatae), d-xylose is converted to d-xylulose by two oxidoreductases in reactions involving the co-factors NAD(P)H and NAD(P)+ through the reductase pathway, where d-xylose is initially reduced to xylitol by NAD(P)H-dependent XR [26–29]. Then xylitol is oxidized to d-xylulose by XDH [26, 28, 30, 31]. d-xylulose is finally phosphorylated to xylulose-5-phosphate by XK [4]. To determine the activities of enzymes involved in the xylose catabolism of T. versicolor, XR, XDH and XK activities were analysed in mycelia grown in MBMC, G11XY11, G6XY11 and G2XY11 media (Table 1) after 18, 66, 114, 234 and 354 h of cultivation at 27°C under hypoxic conditions. XR and XDH activities of mycelia grown in MBMC medium increased exponentially for 66 h, reaching 54.7 and 48.3 μmol min−1 g−1 protein, respectively (Fig. 2). These enzymatic activities of XR and XDH after 66 h of cultivation explain the efficient utilization of xylose (Fig. 2). Between 66 and 354 h of cultivation, XR activity decreased slightly and then remained at ca. 39 μmol min−1 g−1 protein, while XDH activity fell slightly and then increased to 59.5 μmol min−1 g−1 protein (Fig. 2). In addition, the XK activity of mycelia grown in MBMC medium increased exponentially, up to 20.6 μmol min−1 g−1 protein after 18 h of cultivation, then decreased slightly to a constant 17–20 μmol min−1 g−1 protein for up to 354 h growth. The correlations between XR, XDH and XK activities from 0 to 354 h and xylose utilization over the same period (Fig. 2) indicate that T. versicolor can efficiently catabolise xylose in hexose and xylose mixtures and that xylose transport is not inhibited by hexoses. Moreover, this is corroborated by XR, XDH and XK activities measured in mixtures containing different glucose/xylose ratios (Fig. 3b). The activities of XR, XDH and XK all increased in every medium up to 66 h, while they subsequently decreased and remained activity up to 354 h (Fig. 3b). No significant differences were found between the XR, XDH and XK activities in mixtures with different glucose/xylose ratios, with identical xylose concentrations (11 g/l) over 354 h (Fig. 3b), confirming that xylose utilization by T. versicolor is not inhibited by glucose in media where, xylose concentrations are equal or higher than glucose. In addition, significant differences were found between the XR, XDH and XK activities (Fig. 2) in MBMC medium, (glucose and mannose represent 6-carbon 36.7 g/l and xylose represents 5-carbon 11 g/l mixture, Table 1) with respect to different glucose/xylose ratios (Fig. 3b) containing identical xylose concentrations (11 g/l) over 354 h, confirming that xylose utilization by T. versicolor is improved by hexoses in MBMC media (Fig. 2).
Growth and xylose catabolism of Trametes versicolor in the (glucose and xylose mixtures) G11XY11, G6XY11 and G2XY11 media: a biomass (FW fresh weight) production and b top to bottom, XR, XDH and XK activities in mycelia, cultivated under hypoxic conditions. The data represent the mean ± SD from nine samples
Ethanol Production by T. versicolor from Hexoses and Xylose
The industrial S. cerevisiae strain MA-R4 reportedly gives high ethanol yields from a non-sulphuric acid hydrolysate of eucalyptus wood chips [32], and other mixed glucose and xylose carbon sources [33]. Generally, industrial S. cerevisiae strains are superior ethanol producers due to their tolerance of inhibitors and high ethanol productivity under industrial conditions, but to date limited numbers of industrial S. cerevisiae strains have been generated for xylose fermentation [34–39]. In addition, T. versicolor was able to produce 6.94 g/l ethanol from spent sulphite liquor after 36 h of fermentation [40]. To determine the ability of T. versicolor to produce ethanol from hexoses and xylose, the ethanol contents of the MBMC, G11XY11, G6XY11 and G2XY11 media were analysed after 18, 66, 114, 234 and 354 h of cultivation, and XY50 (Table 1) was analysed after 714 h at 27°C, under both anoxic and hypoxic conditions. The ethanol content of the MBMC culture medium gradually increased over 354 h, to a maximum of 20.0 ± 0.79 g/l (Fig. 4a) under hypoxic condition, while under anoxic conditions, ethanol production was not detected, in accordance with reports by [21] that the growth and ethanol production rates of P. tannophilus strongly depend on aeration. However, for commercial use of T. versicolor, we need to improve the fermentation rate by increasing the amount of initial inoculums and bio technologically improvements in xylose-utilizing pathway.
Ethanol fermentation efficiency of Trametes versicolor: a ethanol content in the medium and ADH activity in mycelia cultivated under hypoxic conditions in MBMC medium and b ethanol content in medium and ADH activity in mycelia cultivated under hypoxic conditions. The data represent the mean ± SD from nine samples
Furthermore, P. stipitis, the most efficient natural xylose-fermenting yeast known, produced 0.48 g/g ethanol from xylose when cultured continuously with limited oxygen [41] and ethanol formation from xylose by recombinant S. cerevisie [12] has also been reported. In our study, T. versicolor produced ethanol in media with various xylose/glucose ratios at increasing rates for up to 66 h, and slightly increasing rates for up to 354 h (Fig. 4b), with final ethanol contents in the media of 9.02 g/l ± 0.05 in (G11XY11), 5.4 g/l ± 0.19 (G6XY11) and 1.71 ± 0.08 (G2XY11). The theoretical yield of ethanol from genetically engineered xylose-fermenting yeast was 0.51 g ethanol/g for glucose and 0.51 g ethanol/g for xylose [42]. Therefore, our data suggest that T. versicolor can utilize xylose for ethanol production for up to 354 h in media with various hexose and xylose mixtures under hypoxic conditions. In G11XY11 medium, the ethanol yield was 80% of the theoretical maximum after 354 h under hypoxic conditions, since the actual yield was 9.02 ± 0.05 compared with the theoretical maximum of 11.22 g/l (0.51 g ethanol/g glucose and 0.51 g ethanol/g xylose). Therefore, our results show that T. versicolor produced ethanol from xylose with favourable efficiency when the glucose/xylose ratio was 1:1, under hypoxic conditions.
In addition, XY50 medium ethanol production was detected after 354 h and increased (2.97 ± 0.2 g/l) at 714 h under hypoxic conditions. Furthermore, in XY50 medium no ethanol production was observed over 714 h under anoxic conditions (Fig. 5b). Previous studies have also shown that fungal-mix including T. versicolor can produce ethanol from mixtures of hexoses and xylose [6], and that the fungus P. chrysosporium can degrade lignin in biomass to ethanol by fermenting both hexoses and pentoses [3]. Furthermore, utilization of ethanol by T. versicolor may explain its late ethanol production in XY50 medium (2.97 ± 0.2 g/l) under hypoxic conditions (Fig. 5b). This hypothesis is supported by findings that at high aeration rates the ethanol yield of P. tannophilus decreases, since it respires ethanol in the presence of xylose [21, 43].
The activation of ethanol fermentation is required for recycling NAD+ under limited oxygen conditions, which is essential for maintaining glycolysis. To maintain ethanolic fermentation effectively, ADH and PDC activities are important. Therefore, to determine the contributions of key enzymes to ethanol fermentation by T. versicolor, ADH activity was analysed in mycelia grown in MBMC, G11XY11, G6XY11 and G2XY11 media after 18, 66, 114, 234 and 354 h of cultivation at 27°C under hypoxic conditions. Its ADH activity in MBMC media increased continuously during this time (Fig. 4a), reaching 119.5 ± 4.9 μmol min−1 g−1 protein, and was correlated with ethanol production. Furthermore, ADH activity in the xylose and glucose mixtures increased rapidly over 66 h, but declined rapidly after 114 h in all three mixtures (Fig. 4b). Interestingly, although significant differences were recorded in ethanol production among mycelia cultivated in the different xylose and glucose mixtures over 354 h, surprisingly, their ADH activities were similar and showed similar trends (Fig. 4b). Rapid increases in ADH activity during 66 h of cultivation observed in all three glucose and xylose mixtures were correlated with the ethanol production in the respective cultures (Fig. 4b). In addition, over 66 h with all three glucose and xylose mixtures, biomass production (Fig. 3a) was correlated with XR, XDH and XK activities (Fig. 3b), providing further evidence that T. versicolor expresses xylose catabolic pathways as well as producing ethanol during the active growth stage in all hexose and xylose mixtures used here.
Conclusions
The presented results clearly show that T. versicolor can catabolise xylose and hexoses into ethanol during active growth and under hypoxic conditions. Ethanol production correlates well with biomass production and xylose catabolising enzyme activities in hexose and xylose mixtures used in our study. Furthermore, the genome of T. versicolor is currently being sequenced by JGI [44], bioreactor testing by using spent sulfite liquor and molecular understanding of sugar transporters of T. versicolor is on progress. Thus, species such as T. versicolor are a promising biocatalyst in bio-energy refineries that rely on conversion of lignocellulosic biomass, and may contribute towards fending off the pending energy crisis.
References
Sanchez OJ, Cardona CA (2008) Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol 99:5270–5295
Matsushika A, Inoue H, Kodaki T, Sawayama S (2009) Ethanol production from xylose in engineered Saccharomyces cerevisiae strains: current state and perspectives. Appl Microbiol Biotechnol 84:37–53
Zhang BS (2006) Process for preparing fuel ethanol by using straw fiber materials. Patent CN1880416
Chang SF, Ho NW (1988) Cloning the yeast xylulokinase gene for the improvement of xylose fermentation. Appl Biochem Biotechnol 7:313–318
Perata P, Alpi A (1993) Plant responses to anaerobiosis. Plant Sci 93:1–17
Holmgren M, Sellstedt A (2008) Identification of white-rot and soft-rot fungi increasing ethanol production from spent sulfite liquor in co-culture with Saccharomyces cerevisiae. J App Microbiol 105:134–140
Taherzadeh MJ, Fox M, Hjorth H, Edebo L (2003) Production of mycelium biomass and ethanol from paper pulp sulfite liquor by Rhizopus oryzae. Bioresour Technol 88:167–177
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity of basidiomycetes: application to the identification of mycorrhizae and rust. Mol Ecol 2:113–118
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and application. Academic Press Inc, New York, pp 315–322
Blast search (2010) National Centre for Biotechnological Information. Available at: http://www.ncbi.nlm.gov/blast
Fox A, Morgan SL, Gilbart J (1989) Preparation of alditol acetates and their analysis by gas chromatography (GC) and mass spectrometry (MS). In: Bierman CJ, McGinnis GD (eds) Analysis of carbohydrates by GLC and MS. CRC Press, Florida, pp 87–117
Eliasson A, Christensson CB, Wahlbom FC, Österberg MJ, Thevelein M, Spencer-Martins I et al (2000) Anaerobic xylulose fermentation by recombinant Saccharomyces cerevisiae carrying XYL1, XYL2 and XKS1 in mineral medium chemostat cultures. Appl Environ Microbiol 66:3381–3386
Gibon Y, Blaesing OE, Hannemann J, Carillo P, Höhne M, Hendriks HMJ et al (2004) A Robot-based platform to measure multiple enzyme activities in arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during cycles and in prolonged darkness. Plant Cell 16:3304–3325
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Kirk TK, Farrell RL (1987) Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol 41:465–505
Wu J, Ya-Zhong X, Han-Qing Y (2005) Degradation of lignin in pulp mill wastewaters by white-rot fungi on biofilm. Bioresour Technol 96:1357–1363
Roy BP, Archibald F (1993) Effects of kraft pulp and lignin on Trametes versicolor carbon metabolism. Appl Environ Microbiol 59:1855–1863
Corner EJH (1932) The fruit-body of Polystictus xanthopus. Fr Annals of Botany 46:181
Gilbertson RL (1961) Polyporaceae of the Western United States and Canada 1. Trametes fries. Northweat Science 35:1
Nyanhongo GS, Gubitz G, Sukyai P, Leitner C, Haltrich D, Ludwig R (2007) Oxidoreductases from Trametes spp. In biotechnology: a wealth of catalytic activity. Food Technol Biotechnol 45:250–268
Jeffries TW (1984) Effect of glucose supplements on the fermentation of xylose by Pachysolen tannophilus. Biotechnol Bioeng 27:171–176
Govindaswamy S, Vane LM (2007) Kinetics of growth and ethanol production on different carbon substrates using genetically engineered xylose-fermenting yeast. Bioresour Technol 98:677–685
Bettiga M, Hahn-Hägerdahl B, Gorwa-Grauslund MF (2008) Comparing the xylose reductase/xylitol dehydrogenase and xylose isomerase pathways in arbinose and xylose fermenting Saccharomyces cerevisiae strains. Biotechnol Biofuel 1:16
Zhang M, Eddy C, Deanda K, Finkelstein M, Picataggio S (1995) Metabolic engineering of a pentose metabolism pathway in ethanogenic Zymomonas mobilis. Science 267:240–243
Hamacher T, Becker J, Gardonyi M, Hahn-Hägerdahl B, Boles E (2002) Characterization of the xylose transporting properties of yeast hexose transporters and their influence on xylose utilization. Microbiol 148:2783–2788
Bolen PL, Roth KA, Freer SN (1986) Affinity purifications of aldose reductase and xylitol dehydrogenase from the xylose-fermenting yeast Pachysolen tannophilus. Appl Environ Microbiol 52:660–664
Bruinenberg PM, De Bot PHM, Van Dijken JP, Scheffers WA (1984) NADH-linked aldose reductase: the key to anaerobic fermentation of xylose by yeasts. Appl Microbiol Biotechnol 19:256–260
Rizzi M, Erlemann P, Bui-Thanh NA, Dellweg H (1988) Xylose fermentation by yeasts. 4. Purification and kinetic studies of xylose reductase from Pichia stipitis. Appl Microbiol Biotechnol 29:148–154
Verduyn C, Van Kleef R, Frank J, Schreuder H, Van Dijken JP, Scheffers WA (1985) Properties of the NAD(P)H-dependent xylose reductase from the xylose-fermenting yeast Pichia stipitis. Biochem J 226:669–677
Rizzi M, Harwart K, Erlemann P, Bui-Thanh NA, Dellweg H (1989) Purification and properties of the NAD+-xylitol-dehydrogenase from the yeast Pichia stipitis. J Ferment Bioeng 67:20–24
Wang VW, Jeffries T (1990) Purification and properties of xylitol dehydrogenase from the xylose-fermenting Candida shehatae. Appl Biochem Biotechnol 26:197–206
Matsushika A, Inoue H, Murakami K, Takimura O, Sawayama S (2009) Bio ethanol production performance of five recombinant strains of laboratory and industrial xylose-fermenting Saccharomyces cerevisiae. Bioresour Technol 100:2392–2398
Inoue H, Yano S, Endo T, Sakaki T, Sawayama S (2008) Combining hot-compressed water and ball milling pretreatments to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Biotechnol Biofuel 1:2
Ho NW, Chen Z, Brainard AP, Sedlak M (1999) Successful design and development of genetically engineered Saccharomyces yeasts for effective co-fermentation of glucose and xylose from cellulosic biomass to fuel ethanol. Adv Biochem Eng Biotechnol 65:163–192
Karhumaa K, Wiedemann B, Hahn-Hägerdal B, Boles E, Gorwa-Grauslund MF (2006) Co-utilization of l-arabinose and d-xylose by laboratory and industrial Saccharomyces cerevisiae strains. Microb Cell Fact 5:18
Sedlak M, Ho NW (2004) Production of ethanol from cellulosic biomass hydrolysates using genetically engineered Saccharomyces yeast capable of co-fermenting glucose and xylose. Appl Biochem Biotechnol 114:403–416
Sonderegger M, Jeppsson M, Larsson C, Gorwa-Grauslund MF, Boles E, Olsson L et al (2004) Fermentation performance of engineered and evolved xylose-fermenting Saccharomyces cerevisiae strains. Biotechnol Bioeng 87:90–98
Wahlbom CF, van Zyl WH, Jönsson LJ, Hahn-Hägerdal B, Cordero-Otero RR (2003) Generation of the improved recombinant xylose-utilizing Saccharomyces cerevisiae TMB 3400 by random mutagenesis and physiological comparison with Pichia stipitis CBS 6054. FEMS Yeast Res 3:319–326
Zaldivar J, Borges A, Johansson B, Smits HP, Villas-Bôas SG, Nielsen J et al (2002) Fermentation performance and intracellular metabolite patterns in laboratory and industrial xylose-fermenting Saccharomyces cerevisiae. Appl Microbiol Biotechnol 59:436–442
Holmgreen M, Sellstedt A (2005) Fermentation process, starter culture and growth medium. Patent WO 0548487
Skoog K, Hahn-Hägerdahl B (1990) Effect of oxygenation on xylose fermentation by Pichia stipitis. Appl Environ Microbiol 56:2394–3389
Jin J, Jeffries TW (2004) Stoichiometric network constraints on xylose metabolism by recombinant Saccharomyces cerevisiae. Metab Eng 6:229–238
Maleszka R, Schneider H (1982) Concurrent production and consumption of ethanol by cultures of Pachysolen tannophilus growing on d-xylose. Appl Environ Microbiol 44:909–912
Genome projects (2010) Joint Genome Institute. Available at: http://www.jgi.doe.gov/genome-projects
Acknowledgements
We are grateful to the C. Tryggers Foundation, (Sweden) for financial support of AS and RLK. We are also indebted to Cell wall Lab, UPSC and Kjell Olofsson for the fungus photograph and to all the reviewers of the manuscript for their valuable comments.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
Supplementary Table 1
Sequence amplified by ITS primers (DOC 26 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kudahettige, R.L., Holmgren, M., Imerzeel, P. et al. Characterization of Bioethanol Production from Hexoses and Xylose by the White Rot Fungus Trametes versicolor . Bioenerg. Res. 5, 277–285 (2012). https://doi.org/10.1007/s12155-011-9119-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-011-9119-5