Abstract
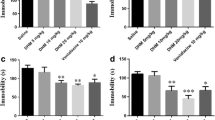
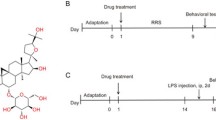
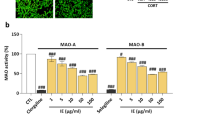
Depression is a debilitating mood disorder that causes persistent feelings of sadness, emptiness, and a loss of joy. However, the clinical efficacy of representative drugs for depression, such as selective serotonin reuptake inhibitors, remains controversial. Therefore, there is an urgent need for more effective therapies to treat depression. Neuroinflammation and the hypothalamic-pituitary-adrenal (HPA) axis are pivotal factors in depression. Inulae Flos (IF), the flower of Inula japonica Thunb, is known for its antioxidant and anti-inflammatory effects. This study explored whether IF alleviates depression in both in vitro and in vivo models. For in vitro studies, we treated BV2 and PC12 cells damaged by lipopolysaccharides or corticosterone (CORT) with IF to investigate the mechanisms of depression. For in vivo studies, C57BL/6 mice were exposed to chronic restraint stress and were administered IF at doses of 0, 100, and 300 mg/kg for 2 weeks. IF inhibited pro-inflammatory mediators, such as nitric oxide, inducible nitric oxide synthase, and interleukins in BV2 cells. Moreover, IF increased the viability of CORT-damaged PC12 cells by modulating protein kinase B, a mammalian target of the rapamycin pathway. Behavioral assessments demonstrated that IF reduced depression-like behaviors in mice. We found that IF reduced the activation of microglia and astrocytes, and regulated synapse plasticity in the mice brains. Furthermore, IF lowered elevated CORT levels in the plasma and restored glucocorticoid receptor expression in the hypothalamus. Collectively, these findings suggest that IF can alleviate depression by mitigating neuroinflammation and recovering dysfunction of the HPA-axis.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Clack S, Ward T (2019) The classification and explanation of depression. Behav Change 36(1):41–55
Organization WH (2008) The global burden of disease: 2004 update. World Health Organization
Coppen A (1967) The biochemistry of affective disorders. Br J Psychiatry 113(504):1237–1264. https://doi.org/10.1192/bjp.113.504.1237
Harmer CJ, Duman RS, Cowen PJ (2017) How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 4(5):409–418. https://doi.org/10.1016/S2215-0366(17)30015-9
Jakobsen JC, Gluud C, Kirsch I (2020) Should antidepressants be used for major depressive disorder? BMJ Evidence-Based Med 25(4):130–130
Moncrieff J, Cohen D (2006) Do antidepressants cure or create abnormal brain states? PLoS Med 3(7):e240. https://doi.org/10.1371/journal.pmed.0030240
Miller AH, Raison CL (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16(1):22–34. https://doi.org/10.1038/nri.2015.5
Hu P, Lu Y, Pan BX, Zhang WH (2022) New insights into the pivotal role of the Amygdala in inflammation-related depression and anxiety disorder. Int J Mol Sci 23(19). https://doi.org/10.3390/ijms231911076
Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M (2015) Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 49:206–215. https://doi.org/10.1016/j.bbi.2015.06.001
Wang H, He Y, Sun Z, Ren S, Liu M, Wang G, Yang J (2022) Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J Neuroinflammation 19(1):132. https://doi.org/10.1186/s12974-022-02492-0
Ting EY, Yang AC, Tsai SJ (2020) Role of Interleukin-6 in depressive disorder. Int J Mol Sci 21(6). https://doi.org/10.3390/ijms21062194
Varghese FP, Brown ES (2001) The hypothalamic-pituitary-adrenal Axis in Major Depressive disorder: a brief primer for Primary Care Physicians. Prim Care Companion J Clin Psychiatry 3(4):151–155. https://doi.org/10.4088/pcc.v03n0401
Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr., Schatzberg AF (2017) HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry 22(4):527–536. https://doi.org/10.1038/mp.2016.120
Holsboer F (2000) The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23(5):477–501. https://doi.org/10.1016/S0893-133X(00)00159-7
De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M (1998) Brain corticosteroid receptor balance in health and disease. Endocr Rev 19(3):269–301. https://doi.org/10.1210/edrv.19.3.0331
Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J, Luo MJ, Tan JH (2015) Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS ONE 10(2):e0117503. https://doi.org/10.1371/journal.pone.0117503
Berger S, Gureczny S, Reisinger SN, Horvath O, Pollak DD (2019) Effect of Chronic Corticosterone Treatment on Depression-Like Behavior and Sociability in Female and Male C57BL/6 N Mice. Cells 8(9). https://doi.org/10.3390/cells8091018
Anacker C, Zunszain PA, Carvalho LA, Pariante CM (2011) The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology 36(3):415–425. https://doi.org/10.1016/j.psyneuen.2010.03.007
Settle EC Jr. (1998) Antidepressant drugs: disturbing and potentially dangerous adverse effects. J Clin Psychiatry 59 Suppl 16:25–30 discussion 40 – 22
Braund TA, Tillman G, Palmer DM, Gordon E, Rush AJ, Harris AWF (2021) Antidepressant side effects and their impact on treatment outcome in people with major depressive disorder: an iSPOT-D report. Transl Psychiatry 11(1):417. https://doi.org/10.1038/s41398-021-01533-1
Noori T, Sureda A, Sobarzo-Sanchez E, Shirooie S (2022) The role of Natural products in treatment of depressive disorder. Curr Neuropharmacol 20(5):929–949. https://doi.org/10.2174/1570159X20666220103140834
Yeung KS, Hernandez M, Mao JJ, Haviland I, Gubili J (2018) Herbal medicine for depression and anxiety: a systematic review with assessment of potential psycho-oncologic relevance. Phytother Res 32(5):865–891. https://doi.org/10.1002/ptr.6033
Li J, Guo X, Luo Z, Wu D, Shi X, Xu L, Zhang Q, Xie C, Yang C (2024) Chemical constituents from the flowers of Inula Japonica and their anti-inflammatory activity. J Ethnopharmacol 318 (Pt B) 117052. https://doi.org/10.1016/j.jep.2023.117052
Qin J-J, Jin H-Z, Zhu J-X, Fu J-J, Zeng Q, Cheng X-R, Zhu Y, Shan L, Zhang S-D, Pan Y-X (2010) New sesquiterpenes from Inula Japonica Thunb. With their inhibitory activities against LPS-induced NO production in RAW264. 7 macrophages. Tetrahedron 66(48):9379–9388
Qin JJ, Jin HZ, Fu JJ, Hu XJ, Wang Y, Yan SK, Zhang WD (2009) Japonicones A-D, bioactive dimeric sesquiterpenes from Inula Japonica Thunb. Bioorg Med Chem Lett 19(3):710–713. https://doi.org/10.1016/j.bmcl.2008.12.043
Qin JJ, Wang LY, Zhu JX, Jin HZ, Fu JJ, Liu XF, Li HL, Zhang WD (2011) Neojaponicone A, a bioactive sesquiterpene lactone dimer with an unprecedented carbon skeleton from Inula Japonica. Chem Commun (Camb) 47(4):1222–1224. https://doi.org/10.1039/c0cc03572f
Wang X, Li Y, Liu W, Shen Y, Lin Z, Nakajima A, Xu J, Guo Y (2023) A polysaccharide from Inula Japonica showing in vivo antitumor activity by interacting with TLR-4, PD-1, and VEGF. Int J Biol Macromol 246:125555. https://doi.org/10.1016/j.ijbiomac.2023.125555
Lu Y, Li Y, Jin M, Yang JH, Li X, Chao GH, Park HH, Park YN, Son JK, Lee E, Chang HW (2012) Inula Japonica extract inhibits mast cell-mediated allergic reaction and mast cell activation. J Ethnopharmacol 143(1):151–157. https://doi.org/10.1016/j.jep.2012.06.015
Kim SH, Ju IG, Kim JH, Eo H, Son SR, Jang DS, Oh MS (2023) Linderae Radix ameliorates cognitive dysfunction by inhibiting neuroinflammation and synaptic damage in Alzheimer’s Disease models. Mol Neurobiol. https://doi.org/10.1007/s12035-023-03544-z
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69. https://doi.org/10.1038/nrn2038
Heras-Sandoval D, Perez-Rojas JM, Hernandez-Damian J, Pedraza-Chaverri J (2014) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26(12):2694–2701. https://doi.org/10.1016/j.cellsig.2014.08.019
Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016) Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22(3):238–249. https://doi.org/10.1038/nm.4050
Jha MK, Jeon S, Suk K (2012) Glia as a link between Neuroinflammation and Neuropathic Pain. Immune Netw 12(2):41–47. https://doi.org/10.4110/in.2012.12.2.41
Guo J, Qiu T, Wang L, Shi L, Ai M, Xia Z, Peng Z, Zheng A, Li X, Kuang L (2022) Microglia loss and astrocyte activation cause dynamic changes in hippocampal [(18)F]DPA-714 uptake in mouse models of Depression. Front Cell Neurosci 16:802192. https://doi.org/10.3389/fncel.2022.802192
Troncoso-Escudero P, Parra A, Nassif M, Vidal RL (2018) Outside in: unraveling the role of Neuroinflammation in the progression of Parkinson’s Disease. Front Neurol 9:860. https://doi.org/10.3389/fneur.2018.00860
Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT (2018) Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y) 4:575–590. https://doi.org/10.1016/j.trci.2018.06.014
Skrzypczak-Wiercioch A, Salat K (2022) Lipopolysaccharide-Induced Model of Neuroinflammation: mechanisms of Action, Research Application and future directions for its use. Molecules 27(17). https://doi.org/10.3390/molecules27175481
O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R (2009) Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 14(5):511–522. https://doi.org/10.1038/sj.mp.4002148
Batista CRA, Gomes GF, Candelario-Jalil E, Fiebich BL, de Oliveira ACP (2019) Lipopolysaccharide-Induced Neuroinflammation as a bridge to Understand Neurodegeneration. Int J Mol Sci 20(9). https://doi.org/10.3390/ijms20092293
Juruena MF, Cleare AJ, Papadopoulos AS, Poon L, Lightman S, Pariante CM (2006) Different responses to dexamethasone and prednisolone in the same depressed patients. Psychopharmacology 189(2):225–235. https://doi.org/10.1007/s00213-006-0555-4
Barnes PJ (2017) Glucocorticosteroids. Handb Exp Pharmacol 237:93–115. https://doi.org/10.1007/164_2016_62
Chourpiliadis C, Aeddula NR (2023) Physiology, glucocorticoids. In: StatPearls. Treasure Island (FL)
Sousa N, Cerqueira JJ, Almeida OF (2008) Corticosteroid receptors and neuroplasticity. Brain Res Rev 57(2):561–570. https://doi.org/10.1016/j.brainresrev.2007.06.007
Neigh GN, Nemeroff CB (2006) Reduced glucocorticoid receptors: consequence or cause of depression? Trends Endocrinol Metab 17(4):124–125. https://doi.org/10.1016/j.tem.2006.03.002
Bridges N, Slais K, Sykova E (2008) The effects of chronic corticosterone on hippocampal astrocyte numbers: a comparison of male and female Wistar rats. Acta Neurobiol Exp (Wars) 68(2):131–138. https://doi.org/10.55782/ane-2008-1682
Khantakova JN, Mutovina A, Ayriyants KA, Bondar NP (2023) Th17 cells, glucocorticoid resistance, and Depression. Cells 12(23). https://doi.org/10.3390/cells12232749
Barnes PJ (2010) Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol 120(2–3):76–85. https://doi.org/10.1016/j.jsbmb.2010.02.018
O’Callaghan JP, Brinton RE, McEwen BS (1989) Glucocorticoids regulate the concentration of glial fibrillary acidic protein throughout the brain. Brain Res 494(1):159–161. https://doi.org/10.1016/0006-8993(89)90156-x
Rozovsky I, Laping NJ, Krohn K, Teter B, O’Callaghan JP, Finch CE (1995) Transcriptional regulation of glial fibrillary acidic protein by corticosterone in rat astrocytes in vitro is influenced by the duration of time in culture and by astrocyte-neuron interactions. Endocrinology 136(5):2066–2073. https://doi.org/10.1210/endo.136.5.7720656
Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J (1994) Neurotoxicity of glucocorticoids in the primate brain. Horm Behav 28(4):336–348. https://doi.org/10.1006/hbeh.1994.1030
Polman JA, Hunter RG, Speksnijder N, van den Oever JM, Korobko OB, McEwen BS, de Kloet ER, Datson NA (2012) Glucocorticoids modulate the mTOR pathway in the hippocampus: differential effects depending on stress history. Endocrinology 153(9):4317–4327. https://doi.org/10.1210/en.2012-1255
Ignacio ZM, Reus GZ, Arent CO, Abelaira HM, Pitcher MR, Quevedo J (2016) New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol 82(5):1280–1290. https://doi.org/10.1111/bcp.12845
Chuang HW, Wei IH, Lin FY, Li CT, Chen KT, Tsai MH, Huang CC (2020) Roles of Akt and ERK in mTOR-Dependent Antidepressant effects of Vanillic Acid. ACS Omega 5(7):3709–3716. https://doi.org/10.1021/acsomega.9b04271
Funding
This research was supported by grants from the National Research Foundation of Korea, funded by the Korean government (2022M3A9B6017813).
Author information
Authors and Affiliations
Contributions
Jin Se Kim: Conceptualization, Investigation, Writing – Original Draft Preparation; Jin Hee Kim: Investigation, Writing – Original Draft Preparation; Hyeyoon Eo: Writing – Review & Editing; In Gyoung Ju: Writing – Review & Editing; So-Ri Son: Investigation; Ji-Woon Kim: Supervision; Dae Sik Jang: Supervision; Myung Sook Oh: Conceptualization, Supervision, Writing – Review & Editing.
Corresponding author
Ethics declarations
Ethical Approval
All animal studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition (National Institutes of Health, 2011), and approved by the Animal Care and Use Guidelines of Kyung Hee University, Seoul, Republic of Korea (approval number: KHSASP-23–451).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J.S., Kim, J.H., Eo, H. et al. Inulae Flos has Anti-Depressive Effects by Suppressing Neuroinflammation and Recovering Dysfunction of HPA-axis. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04094-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04094-8