Abstract
Chronic stress is an important factor to several physical and mental diseases. Ishige foliacea, an edible brown seaweed, has various biological properties; however, its role in alleviating stress-induced depression remains unclear. Thus, the antidepressant-like effects of I. foliacea ethanolic extract (IE) were investigated using corticosterone (CORT)-treated HT22 cells and CORT-induced depressive mice. IE significantly reduced the production of reactive oxygen species (ROS) and inhibited monoamine oxidase (MAO) activity and protein expression; furthermore, it up-regulated the extracellular signal-regulated kinase (ERK)-cAMP response element-binding protein (CREB)-brain derived neurotrophic factor (BDNF) signaling pathway. In the CORT-induced depressive mice, IE administration (500 mg/kg, bodyweight) exerted antidepressant effects according to behavioral tests. In addition, IE activated the ERK-CREB-BDNF signaling pathway owing to CORT exposure in the hippocampus. Therefore, IE inhibited ROS production and MAO activity and improved depressive behaviors caused by stress hormones, which demonstrates the therapeutic potential of IE in the treatment of depression.
Similar content being viewed by others
Introduction
Depression has become a major health issue that affects 280 million people worldwide and causes severe health, social, and economic burdens [1]. The primary features of depression have been reported to be hypothalamus-pituitary-adrenal axis hyperactivity owing to stress, high glucocorticoid levels, and impaired regulation of feedback inhibition [2]. In this regard, antidepressants are the first-line therapy for patients with less severe cases; however, multiple concerns remain about their effectiveness, efficiency, and side effects [3]. Hence, effective alternative treatment options are desired.
Marine algae frequently produce an abundance of bioactive compounds. In particular, brown algae contain various constituents such as phlorotannins, pigments, and fucoidans [4]. Ishige foliacea is an edible brown alga that mainly inhabits Jeju Island, South Korea. Many biological activities of I. foliacea, including antioxidant, anti-inflammatory, and hypoglycemic effects have been reported [5, 6]. In addition, previous study has reported that an I. foliacea ethanol extract alleviates scopolamine-induced memory impairment by protecting oxidative stress-induced cellular damage and activating the extracellular signal-regulated kinase (ERK) –brain-derived neurotrophic factor (BDNF)–tropomyosin receptor kinase B (TrkB) pathway in the hippocampus [7]. However, despite numerous pharmacological studies, the effects of I. foliacea on depression have not been elucidated. Therefore, in the present study, these effects and their potential mechanisms were evaluated using the I. foliacea ethanol extract (IE) in HT22 cells and mice with depression induced by a stress hormone.
Materials and methods
Preparation of I. foliacea ethanol extract
I. foliacea from Jeju Island (South Korea) were washed, dried, and extracted 10 times with 70% (v/v) ethanol/water at 50 °C for 24 h. After filtered, the extracts were subsequently lyophilized and air-dried.
Monoamine oxidase enzyme activity
Monoamine oxidase (MAO) activity was measured using an Amplex Red Monoamine Oxidase Assay Kit (Invitrogen, Carlsbad, CA, USA). Fluorescence was measured using a fluorescence microplate reader (Molecular Device, Sunnyvale, CA, USA) at excitation = 550 nm and emission = 590 nm, respectively.
Cell culture and cell viability
The mouse hippocampal neuronal HT22 cells (ATCC, Rockville, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin in a humidified incubator at 37℃ under 5% CO2 and 95% air. Cell viability was assessed by MTT [3-(4,5-dime thylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay as described by Um et al. [8].
Reactive oxygen species measurements
To measure the reactive oxygen species (ROS) production, 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA) staining assay was performed as per the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). HT22 cells (1 × 104 cells/well) were co-treated with 200 μM corticosterone (CORT) and IE (10 or 100 μg/mL) for 24 h. Using a fluorescence microscope (Olympus, Shinjuku, Tokyo, Japan), DCFH-DA fluorescence was captured.
Animals and treatments
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Korea Food Research Institute (KFRI-M-17064). Male ICR mice (5 weeks of age, 21–25 g) were obtained from KOATECH Animal Inc. (Pyeongteak, South Korea) and housed under constant conditions on a 12-h light/dark cycle at 23 ± 1 °C and 55 ± 5% humidity. After 1-week adaption, the mice received repeat intraperitoneal (i.p.) injections of CORT (40 mg/kg bodyweight (B.W.)) to induce depressive-like behavior. Fifty mice were divided randomly into five groups: (1) Vehicle+Vehicle (NOR), (2) CORT+Vehicle (CORT+VEH), (3) CORT+fluoxetine (FLU) at 20 mg/kg B.W. (CORT+FLU), (4) CORT+Hypericum perforatum (St John’s wort) extract (SE) at 300 mg/kg B.W. (CORT+SE), and (5) CORT+IE at 500 mg/kg B.W. (CORT+IE). In this study, FLU and SE were used as positive controls. All dosages were based on previously published studies [7, 9, 10]. After the mice were anesthetized with isoflurane, brain tissues were subsequently isolated and stored at −80 °C. The experimental scheme is presented in Fig. 3a.
Behavioral tests
Tail suspension test
The tail suspension test (TST) was performed according to a previously described method [11]. The immobility time was measured for 6 min using a TST apparatus (BioSeb, Chaville, France).
Forced swimming test
The forced swimming test (FST) was performed according to a previously described method [12]. The immobility time was recorded for 6 min and analyzed by SMART 3.0 software (Panlab SL, Barcelona, Spain).
Western blotting
Protein was extracted from HT22 cells and hippocampal tissues using a RIFA buffer with a cocktail inhibitor and quantified using BCA assay kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Proteins were separated using 10–12% SDS-PAGE and transferred to PVDF membranes (Bio-Rad, Hercules, CA, USA). After blocking, membranes were incubated with primary antibodies (MAO-A, MAO-B, ERK, p-ERK, CREB, p-CREB, BDNF, and α-tubulin) overnight at 4 °C (Cell signaling Technology, Danvers, Massachusetts, USA). Using a chemiluminescence imaging system (Vilber Lourmat, France), membranes were visualized. Relative protein densities were measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are presented as mean ± standard deviation. Comparisons among groups were performed using one-way analysis of variance, followed by Tukey’s post-hoc test using Prism 9 (GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was set at p < 0.05.
Results and discussion
IE attenuated CORT-induced ROS production via inhibition of MAO in HT22 cells
Stress hormone could increase ROS production by activation of MAO enzyme in the brain [13]. Previous studies have reported that MAO is an crucial source of hydrogen peroxide [14]. Damier et al. [15] reported that MAO-catalyzed ROS production caused increased cellular damage. Hence, MAO inhibition is directly associated with the scavenging capacity of ROS; therefore, ROS levels in HT22 neuronal cells were assessed in the present study using DCFH-DA. When HT22 cells were treated with IE, the cell viability did not change. Figure 1a shows, after exposure to 200 μM CORT for 24 h, intracellular ROS levels significantly increased to 147% compared to those from VEH-only treated HT22 cells (p < 0.01). However, this elevated ROS production was significantly decreased by treatment with IE (10 or 100 μg/ml).
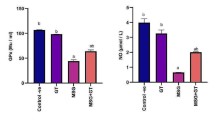
a Effects of IE on the ROS production in CORT-treated HT22 cells. DCFH-DA fluorescence images and intensity. HT22 cells were co-treated with CORT (200 μM) and IE (10 or 100 μg/mL) for 24 h. After staining using DCFH-DA (5 μM) for 45 min, fluorescence images were captured. b-c Effects of IE on the MAO enzyme activity and protein expression. b IE inhibited both MAO-A and MAO-B activity at concentrations of 1–100 μg/mL. c Immunoblot showing MAO protein expression. Data are presented as mean ± standard deviation. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. CTL; *p < 0.05, **p < 0.01 vs. CORT-only treated cells
Next, we confirmed whether IE regulated MAO activities as an underlying mechanism of CORT-induced ROS production. MAO is strongly related to stress-induced depression because it catalyzes the oxidative degradation of neurotransmitters [16]. Several studies have reported this dysregulation of neurotransmitters by MAO in the brains of patients with depression [17, 18]. Thus, MAO inhibition provides an important treatment for depression. In the present study, IE remarkably inhibited both MAO-A and MAO-B activities at all concentrations (1–100 μg/mL) compared with those of the control (Fig. 1b), suggesting that IE can prevent oxidative stress by regulating MAO activity. Additionally, whether IE also regulated MAO protein expression was investigated. The immunoblotting data revealed that MAO-A and MAO-B protein expression increased following 24 h of exposure to 200 μM CORT compared to those of the control in HT22 cells. However, treatment with IE remarkably inhibited the CORT-mediated increase in MAO expressions (Fig. 1c). Therefore, the results suggest that IE modulates CORT-induced ROS production by suppressing MAO activities and protein expressions.
IE up-regulated the ERK-CREB-BDNF signaling pathway in CORT-treated HT22 cells
BDNF is a key neurotrophic factor and plays an crucial role in neurodegenerative diseases and depression [19]. CREB is the transcription factor of BDNF and also plays an important role in depression [20]. ERK, which is upstream to CREB, controls neurogenesis and activates in response to stress [21]. Therefore, the ERK-CREB-BDNF signaling pathway is associated with the neurogenesis, synaptic plasticity, and pathology of depression [22]. Additionally, several studies have reported that this signaling was downregulated in depressive animal models [23, 24]. Based on these facts, in present study, the regulatory effects of IE on the ERK-CREB-BDNF signaling pathway in response to CORT were investigated. Figure 2 shows that BDNF expression was down-regulated following CORT treatment in HT22 cells, which was reversed by IE treatment. Moreover, ERK and CREB phosphorylation significantly decreased in the HT22 cells treated with CORT, but IE partially reversed this reduction. Several antidepressants, including drugs, natural products, and phytochemicals, have upregulated BDNF, CREB, and ERK expression [25, 26]. For example, phlorotannins, which are prevalent in brown algae extracts, including that of I. foliacea, promote neuronal plasticity and upregulate BDNF and CREB mRNA levels in stress hormone-treated neuroblastoma cells [27]. As previous studies have reported, a decrease in the activation of ERK-CREB-BDNF signaling pathways leads to depressive-like behaviors in rodents; thus, several antidepressants, such as FLU, can alleviate these symptoms by upregulating this pathway [28]. Therefore, IE may up-regulate the reduction of ERK-CREB-BDNF signaling pathway induced by CORT.
Effects of IE on the ERK-CREB-BDNF signaling pathway in CORT-treated HT22 cells. Immunoblot representing the ERK-CREB-BDNF signaling pathway related-protein expression. Data are presented as mean ± standard deviation. ##p < 0.01, ###p < 0.001 vs. CTL; **p < 0.01, ***p < 0.001 vs. CORT-only treated cells
IE improved depressive-like behaviors in CORT-treated mice
To determine the effects of IE on depression in CORT injection induced-depressive animal models, behavioral tests (TST and FST) were conducted based on our in vitro findings. CORT treatment can induce depressive-like behaviors in rodents [29]. For evaluation of depression pathology in rodents, the TST and FST are powerful tools due to its good reliability and predictive validity [30]. The immobility during the TST and FST has been reported to represent depression-like phenotypes and can be attenuated by antidepressant treatment [31]. FLU is a widely used antidepressant, and SE is a commonly used alternative medicines for mild depression [32, 33]. Figure 3b and c represent that the CORT+VEH group had an increase in immobility time during the TST and FST in comparison with those of the NOR group. However, IE administration significantly decreased immobility times during both tests, similar to results with FLU and SE. Therefore, IE has antidepressant-like effects in CORT-treated mice.
Effects of IE on the CORT-induced depressive-like behaviors in mice. a Experimental schedule. Mice were exposed to CORT (40 mg/kg/day, i.p.) for 14 days with FLU (20 mg/kg/day, per oral (p.o.)), SE (300 mg/kg/day, p.o.), or IE (500 mg/kg/day, p.o.). Immobility time during the b TST and c FST (n = 10 per group). Data are presented as mean ± standard deviation. #p < 0.05, ##p < 0.01 vs. NOR group; **p < 0.01, ***p < 0.001 vs. CORT-treated mice
To further examine the underlying mechanisms of the antidepressant effects of IE, the ERK-CREB-BDNF signaling pathway in the hippocampus was investigated via immunoblotting analysis. As expected, CORT remarkably reduced the hippocampal p-ERK, p-CREB, and BDNF expressions in the CORT+VEH group in comparison with those in the NOR group (Fig. 4). Interestingly, we did not find change in the CREB phosphorylation, with the exception of significant increase of the ERK phosphorylation and BDNF protein expression in the hippocampus after chronic IE administration. However, these results suggested that other factors might be responsible for the modification found in depressive-like behaviors.
Effects of IE on the ERK-CREB-BDNF signaling pathway in the hippocampus of CORT-treated mice. Immunoblot representing the ERK-CREB-BDNF signaling pathway related-protein expression. Data are presented as mean ± standard deviation. ##p < 0.01, ###p < 0.001 vs. NOR group; ***p < 0.001 vs. CORT-treated mice
In summary, our study demonstrates that IE inhibited MAO activity and ROS production. Additionally, IE improved CORT-induced depressive symptoms by enhancing the ERK-CREB-BDNF signaling pathway in both HT22 cells and the hippocampus of mice. However, further studies are needed to identify the active ingredient in IE and investigate its detailed mechanisms, such as examining neurotransmitters, genes associated with stress-susceptibility, and synaptic plasticity. Nevertheless, IE may provide a potential agent for preventing depression.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BDNF:
-
Brain derived neurotrophic factor
- CORT:
-
Corticosterone
- CREB:
-
cAMP response element-binding protein
- CTL:
-
Control
- DCFH-DA:
-
2',7'-Dichlorodihydrofluorescein diacetate
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ERK:
-
Extracellular signal-regulated kinase
- FBS:
-
Fetal bovine serum
- FLU:
-
Fluoxetine
- FST:
-
Forced swimming test
- IE:
-
Ishige foliacea ethanol extract
- MAO:
-
Monoamine oxidase
- ROS:
-
Reactive oxygen species
- SE:
-
Hypericum perforatum (St John’s wort) extract
- TrkB:
-
Tropomyosin receptor kinase B
- TST:
-
Tail suspension test
References
World Health Organization (2021) Depression. https://www.who.int/news-room/fact-sheets/detail/depression. Accessed 21 Oct 2021.
Juruena MF, Cleare AJ, Bauer ME, Pariante CM (2003) Molecular mechanisms of glucocorticoid receptor sensitivity and relevance to affective disorders. Acta Neuropsychiatr 15(6):354–367
Arroll B, Macgillivray S, Ogston S, Reid I, Sullivan F, Williams B, Crombie I (2005) Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: a meta-analysis. Ann Fam Med 3(5):449–456
Lee S-H, Kang S-M, Ko S-C, Lee D-H, Jeon Y-J (2012) Octaphlorethol A, a novel phenolic compound isolated from a brown alga, Ishige foliacea, increases glucose transporter 4-mediated glucose uptake in skeletal muscle cells. Biochem Biophys Res Commun 420(3):576–581
Lee S-H, Kang S-M, Ko S-C, Kang M-C, Jeon Y-J (2013) Octaphlorethol A, a novel phenolic compound isolated from Ishige foliacea, protects against streptozotocin-induced pancreatic β cell damage by reducing oxidative stress and apoptosis. Food Chem Toxicol 59:643–649
Manzoor Z, Mathema VB, Chae D, Kang H-K, Yoo E-S, Jeon Y-J, Koh Y-S (2013) Octaphlorethol A inhibits the CpG-induced inflammatory response by attenuating the mitogen-activated protein kinase and NF-κB pathways. Biosci Biotechnol Biochem 77(9):1970–1972
Kim TE, Son HJ, Lim DW, Yoon M, Lee J, Kim YT, Han D, Lee C, Um MY (2020) Memory-enhancing effects of Ishige foliacea extract: In vitro and in vivo study. J Food Biochem 44(4):e13162
Um MY, Ahn JY, Kim MK, Ha TY (2012) Sesaminol glucosides protect β-amyloid induced apoptotic cell death by regulating redox system in SK-N-SH cells. Neurochem Res 37(4):689–699
Badr AM, Attia HA, Al-Rasheed N (2020) Oleuropein reverses repeated corticosterone-induced depressive-like behavior in mice: evidence of modulating effect on biogenic amines. Sci Rep 10(1):1–10
Lim DW, Han T, Um MY, Yoon M, Kim T-E, Kim YT, Han D, Lee J, Lee CH (2019) Administration of Asian herb bennet (Geum japonicum) extract reverses depressive-like behaviors in mouse model of depression induced by corticosterone. Nutrients 11(12):2841
Cryan JF, Mombereau C, Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29(4–5):571–625
Yankelevitch-Yahav R, Franko M, Huly A, Doron R (2015) The forced swim test as a model of depressive-like behavior. J Vis Exp 97:e52587
Mallajosyula JK, Kaur D, Chinta SJ, Rajagopalan S, Rane A, Nicholls DG, Di Monte DA, Macarthur H, Andersen JK (2008) MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PLoS ONE 3(2):e1616
Maurel A, Hernandez C, Kunduzova O, Bompart G, Cambon C, Parini A, Francés B (2003) Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am J Physiol Heart Circ Physiol 284(4):H1460–H1467
Damier P, Kastner A, Agid Y, Hirsch EC (1996) Does monoamine oxidase type B play a role in dopaminergic nerve cell death in Parkinson’s disease? Neurology 46(5):1262–1262
Edmondson DE (2014) Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: biological implications. Curr Pharm Des 20(2):155–160
Duncan J, Johnson S, Ou X-M (2012) Monoamine oxidases in major depressive disorder and alcoholism. Drug Discov Ther 6(3):112–122
Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, Young T, Praschak-Rieder N, Wilson AA, Houle S (2006) Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry 63(11):1209–1216
You J, Yuan Y, Zhang Z, Zhang X, Li H, Qian Y (2010) A preliminary association study between brain-derived neurotrophic factor (BDNF) haplotype and late-onset depression in mainland Chinese. J Affect Disord 120(1–3):165–169
Oury F, Yadav VK, Wang Y, Zhou B, Liu XS, Guo XE, Tecott LH, Schutz G, Means AR, Karsenty G (2010) CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. Genes Dev 24(20):2330–2342
Leem Y-H, Yoon S-S, Kim Y-H, Jo SA (2014) Disrupted MEK/ERK signaling in the medial orbital cortex and dorsal endopiriform nuclei of the prefrontal cortex in a chronic restraint stress mouse model of depression. Neurosci Lett 580:163–168
Li W, Zhu Y, Saud SM, Guo Q, Xi S, Jia B, Jiao S, Yang X, Lu J, Song S (2017) Electroacupuncture relieves depression-like symptoms in rats exposed to chronic unpredictable mild stress by activating ERK signaling pathway. Neurosci Lett 642:43–50
Dong H, Cong W, Guo X, Wang Y, Tong S, Li Q, Li C (2019) β-asarone relieves chronic unpredictable mild stress induced depression by regulating the extracellular signal-regulated kinase signaling pathway. Exp Ther Med 18(5):3767–3774
Jia Z, Yang J, Cao Z, Zhao J, Zhang J, Lu Y, Chu L, Zhang S, Chen Y, Pei L (2021) Baicalin ameliorates chronic unpredictable mild stress-induced depression through the BDNF/ERK/CREB signaling pathway. Behav Brain Res 414:113463
Lu J, Zhou H, Meng D, Zhang J, Pan K, Wan B, Miao Z (2020) Tanshinone IIA improves depression-like behavior in mice by activating the ERK-CREB-BDNF signaling pathway. Neuroscience 430:1–11
Yu H, Shao S, Xu J, Guo H, Zhong Z, Xu J (2022) Persimmon leaf extract alleviates chronic social defeat stress-induced depressive-like behaviors by preventing dendritic spine loss via inhibition of serotonin reuptake in mice. Chin Med 17(1):1–20
Gite S, Ross RP, Kirke D, Guihéneuf F, Aussant J, Stengel DB, Dinan TG, Cryan JF, Stanton C (2019) Nutraceuticals to promote neuronal plasticity in response to corticosterone-induced stress in human neuroblastoma cells. Nutr Neurosci 22(8):551–568
Qi X, Lin W, Li J, Li H, Wang W, Wang D, Sun M (2008) Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis 31(2):278–285
Li H, Li J, Zhang T, Xie X, Gong J (2022) Antidepressant effect of Jujuboside A on corticosterone-induced depression in mice. Biochem Biophys Res Commun 620:56–62
Petit-Demouliere B, Chenu F, Bourin M (2005) Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology 177(3):245–255
Bai Y, Song L, Dai G, Xu M, Zhu L, Zhang W, Jing W, Ju W (2018) Antidepressant effects of magnolol in a mouse model of depression induced by chronic corticosterone injection. Steroids 135:73–78
Pirotta M, Willis K, Carter M, Forsdike K, Newton D, Gunn J (2014) ‘Less like a drug than a drug’: the use of St John’s wort among people who self-identify as having depression and/or anxiety symptoms. Complement Ther Med 22(5):870–876
Magni LR, Purgato M, Gastaldon C, Papola D, Furukawa TA, Cipriani A, Barbui C (2013) Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004185.pub3
Acknowledgements
Not applicable.
Funding
This research was supported by the Main Research Program of the Korea Food Research Institute (KFRI), which is funded by the Ministry of Science and ICT (E0210201-02); the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET), which is funded by the Ministry of Agriculture, Food, and Rural Affairs (116036-03); and the Korea Institute of Marine Science & Technology Promotion (KIMST), which is funded by the Ministry of Oceans and Fisheries (20220488).
Author information
Authors and Affiliations
Contributions
MYU designed the experiment. MK. SK, and SC performed the experiments and analyzed data. MK and MYU wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, M., Kwon, S., Cho, S. et al. Ishige foliacea ameliorates depressive-like behaviors in stress hormone treated mice. Appl Biol Chem 65, 86 (2022). https://doi.org/10.1186/s13765-022-00757-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-022-00757-z