Abstract
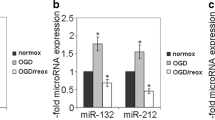
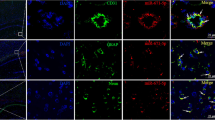
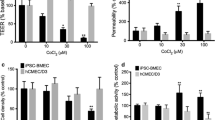
Stroke is a life-threatening medical condition across the world that adversely affects the integrity of the blood–brain barrier (BBB). The brain microvascular endothelial cells are the important constituent of the BBB. These cells line the blood vessels and form a semipermeable barrier. Disruptions in adherens junction and tight junction proteins of brain microvascular endothelial cells compromise the integrity of BBB. The Vascular Endothelial (VE)-cadherin is an integral adherens junction protein required for the establishment and maintenance of the endothelial barrier integrity. This study aims to investigate the role of miRNA in hypoxia-induced endothelial barrier disruption. In this study, brain endothelial cells were exposed to hypoxic conditions for different time points. Western blotting, overexpression and knockdown of miRNA, real-time PCR, TEER, and sodium fluorescein assay were used to examine the effect of hypoxic conditions on brain endothelial cells. Hypoxic exposure was validated using HIF-1α protein. Exposure to hypoxic conditions resulted to a significant decrease in endothelial barrier resistance and an increase in sodium fluorescein migration across the endothelial barrier. Reduction in endothelial barrier resistance demonstrated compromised barrier integrity, whereas the increase in migration of sodium fluorescein across the barrier indicated the increase in barrier permeability. The present study revealed microRNA-101 decreases the expression of VE-cadherin and claudin-5 in brain endothelial cells exposed to the hypoxic conditions.






Similar content being viewed by others
Data Availability
All data generated in this study are available from the corresponding author on reasonable request.
References
Abbott NJ et al (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37(1):13–25
Daneman R (2012) The blood-brain barrier in health and disease. Ann Neurol 72(5):648–672
Dejana E, Orsenigo F, Lampugnani MG (2008) The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 121(13):2115–2122
Greene C, Hanley N, Campbell M (2019) Claudin-5: gatekeeper of neurological function. Fluids Barriers CNS 16(1):3
Harris ES, Nelson WJ (2010) VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Curr Opin Cell Biol 22(5):651–658
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14(3):133–150
Yang Y, Rosenberg GA (2011) Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42(11):3323–3328
O’Donnell MJ et al (2010) Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 376(9735):112–123
Page S, Munsell A, Al-Ahmad AJ (2016) Cerebral hypoxia/ischemia selectively disrupts tight junctions complexes in stem cell-derived human brain microvascular endothelial cells. Fluids Barriers CNS 13(1):16
Feigin VL et al (2019) Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(5):459–480
Singh SK et al (2008) MicroRNAs–micro in size but macro in function. Febs J 275(20):4929–4944
Sabirzhanov B et al (2014) Downregulation of miR-23a and miR-27a following experimental traumatic brain injury induces neuronal cell death through activation of proapoptotic Bcl-2 proteins. J Neurosci 34(30):10055–10071
Burek M et al (2019) Hypoxia-induced MicroRNA-212/132 alter blood-brain barrier integrity through inhibition of tight junction-associated proteins in human and mouse brain microvascular endothelial cells. Transl Stroke Res 10(6):672–683
Chen K et al (2012) MicroRNA-101 mediates the suppressive effect of laminar shear stress on mTOR expression in vascular endothelial cells. Biochem Biophys Res Commun 427(1):138–142
Xi T et al (2017) MicroRNA-126-3p attenuates blood-brain barrier disruption, cerebral edema and neuronal injury following intracerebral hemorrhage by regulating PIK3R2 and Akt. Biochem Biophys Res Commun 494(1–2):144–151
Cerutti C et al (2016) MicroRNA-155 contributes to shear-resistant leukocyte adhesion to human brain endothelium in vitro. Fluids Barriers CNS 13(1):1–7
Chio CC et al (2013) MicroRNA-210 targets antiapoptotic Bcl-2 expression and mediates hypoxia-induced apoptosis of neuroblastoma cells. Arch Toxicol 87(3):459–468
Ge X-T et al (2014) miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci Rep 4(1):1–11
Sun L et al (2018) miR-23b improves cognitive impairments in traumatic brain injury by targeting ATG12-mediated neuronal autophagy. Behav Brain Res 340:126–136
Xi T et al (2018) miR-27a-3p protects against blood–brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. J Biol Chem 293(52):20041–20050
Chen Q et al (2020) miR-101–3p induces vascular endothelial cell dysfunction by targeting tet methylcytosine dioxygenase 2. Acta Biochim Biophys Sin 52(2):180–191
Song J et al (2018) miR-1303 regulates BBB permeability and promotes CNS lesions following CA16 infections by directly targeting MMP9. Emerg Microbes Infect 7(1):1–15
Wang J et al (2020) MiRNA-125a-5p attenuates blood–spinal cord barrier permeability under hypoxia in vitro. Biotech Lett 42(1):25–34
Li B et al (2020) MiRNA-210 induces microglial activation and regulates microglia-mediated neuroinflammation in neonatal hypoxic-ischemic encephalopathy. Cell Mol Immunol 17(9):976–991
Bhardwaj U, Singh SK (2021) Zika virus NS1 suppresses VE-cadherin and Claudin-5 via hsa-miR-101-3p in human brain microvascular endothelial cells. Mol Neurobiol 58(12):6290–6303
Kulshreshtha R et al (2007) Regulation of microRNA expression: the hypoxic component. Cell Cycle 6(12):1426–1431
Chen SL et al (2020) JAK/STAT signaling pathway-mediated microRNA-181b promoted blood-brain barrier impairment by targeting sphingosine-1-phosphate receptor 1 in septic rats. Ann Transl Med 8(21):1458
Li D et al (2017) miR-285–Yki/Mask double-negative feedback loop mediates blood–brain barrier integrity in Drosophila. Proc Natl Acad Sci U.S.A. 114(12):E2365–E2374
Bai Y et al (2016) Silencing microRNA-143 protects the integrity of the blood-brain barrier: implications for methamphetamine abuse. Sci Rep 6(1):1–15
Bhardwaj U, Singh SK (2023) Zika virus NS1 suppresses VE-cadherin via hsa-miR-29b-3p/DNMT3b/MMP-9 pathway in human brain microvascular endothelial cells. Cell Signal 106:110659
Mishra R, Singh SK (2013) HIV-1 Tat C modulates expression of miRNA-101 to suppress VE-cadherin in human brain microvascular endothelial cells. J Neurosci 33(14):5992
Wang Y et al (2018) MicroRNA-130a regulates cerebral ischemia–induced blood–brain barrier permeability by targeting Homeobox A5. FASEB J 32(2):935–944
Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19(12):1584–1596
Engelhardt B, Liebner S (2014) Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res 355(3):687–699
Giannotta M, Trani M, Dejana E (2013) VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell 26(5):441–454
Semenza GL (2001) Hypoxia-inducible factor 1: control of oxygen homeostasis in health and disease. Pediatr Res 49(5):614–617
Bartoszewski R et al (2019) Primary endothelial cell-specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. FASEB J 33(7):7929–7941
Engelhardt S et al (2014) Hypoxia selectively disrupts brain microvascular endothelial tight junction complexes through a hypoxia-inducible factor-1 (HIF-1) dependent mechanism. J Cell Physiol 229(8):1096–1105
Patak P et al (2011) The ATP-binding cassette transporters ABCB1 and ABCC1 are not regulated by hypoxia in immortalised human brain microvascular endothelial cells. Exp Transl Stroke Med 3:12
Baldea I et al (2018) Effects of different hypoxia degrees on endothelial cell cultures—time course study. Mech Ageing Dev 172:45–50
Koto T et al (2007) Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol 170(4):1389–1397
Zhang H et al (2018) Bone morphogenetic protein-7 inhibits endothelial-mesenchymal transition in pulmonary artery endothelial cell under hypoxia. J Cell Physiol 233(5):4077–4090
Walsh TG et al (2011) Stabilization of brain microvascular endothelial barrier function by shear stress involves VE-cadherin signaling leading to modulation of pTyr-occludin levels. J Cell Physiol 226(11):3053–3063
Gavard J, Gutkind JS (2008) VE-cadherin and claudin-5: it takes two to tango. Nat Cell Biol 10(8):883–885
Taddei A et al (2008) Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol 10(8):923–934
Page S, Munsell A, Al-Ahmad AJ (2016) Cerebral hypoxia/ischemia selectively disrupts tight junctions complexes in stem cell-derived human brain microvascular endothelial cells. Fluids Barriers CNS 13(1):16
Feigin VL et al (2021) Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 20(10):795–820
Liu LZ et al (2011) MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS One 6(4):e19139
Zhang M et al (2023) miR-101-3p improves neuronal morphology and attenuates neuronal apoptosis in ischemic stroke in young mice by downregulating HDAC9. Transl Neurosci 14(1):20220286
Cao S et al (2019) The upregulation of miR-101 promotes vascular endothelial cell apoptosis and suppresses cell migration in acute coronary syndrome by targeting CDH5. Int J Clin Exp Pathol 12(9):3320–3328
Kim JH et al (2014) Hypoxia-responsive microRNA-101 promotes angiogenesis via heme oxygenase-1/vascular endothelial growth factor axis by targeting cullin 3. Antioxid Redox Signal 21(18):2469–2482
Pang J et al (2020) The effect of MicroRNA-101 on angiogenesis of human umbilical vein endothelial cells during hypoxia and in mice with myocardial infarction. Biomed Res Int 2020:5426971
Smits M et al (2011) Down-regulation of miR-101 in endothelial cells promotes blood vessel formation through reduced repression of EZH2. PLoS One 6(1):e16282
Long JM, Lahiri DK (2011) MicroRNA-101 downregulates Alzheimer’s amyloid-β precursor protein levels in human cell cultures and is differentially expressed. Biochem Biophys Res Commun 404(4):889–895
Qiang J et al (2020) miR-34a regulates the activity of HIF-1a and P53 signaling pathways by promoting GLUT1 in genetically improved farmed tilapia (GIFT, Oreochromis niloticus) under hypoxia stress. Front Physiol 11:670
Acknowledgements
The authors acknowledge the funding support provided through the Institutions of Eminence (IoE-6031) Scheme of Banaras Hindu University, Varanasi, and Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. Astha Shukla and Apoorva acknowledge the financial assistance provided by UGC, India, through NON-NET fellowship. Utkarsh Bhardwaj is a recipient of the Indian Council of Medical Research (ICMR)-Senior Research Fellowship. The authors also acknowledge Ms. Neha Pandey for her critical help during manuscript drafting.
Funding
This work was financially supported by the funding received by the Department of Biotechnology, Ministry of Science and Technology, Govt. of India, (BT/PR23625/MED/122/77/2017).
Author information
Authors and Affiliations
Contributions
AS and UB did the experiments and analyzed the data, and SKS conceptualized the hypothesis and experimental design. AS, UB and Apoorva drafted the manuscript. PS provided the hCMEC/D3 cells and shared his knowledge as a Co-Investigator. SKS supervised the work and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shukla, A., Bhardwaj, U., Apoorva et al. Hypoxia-Induced miR-101 Impairs Endothelial Barrier Integrity Through Altering VE-Cadherin and Claudin-5. Mol Neurobiol 61, 1807–1817 (2024). https://doi.org/10.1007/s12035-023-03662-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03662-8