Abstract
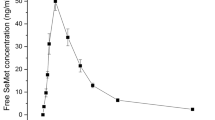
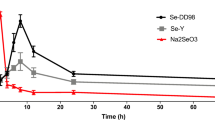
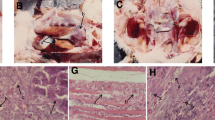
The purpose of this research is to investigate the absorption, distribution, excretion, and pharmacokinetics of selenite in rats after intragastric administration, and thus illustrate the efficiency of selenium (Se) supplementation. After a single gavage of sodium selenite, a concentration of Se in plasma and tissues was determined by inductively coupled plasma mass spectrometry (ICP-MS) at different time points. Through fitting the data with the metabolic kinetic model, the corresponding kinetic parameters were determined for plasma and tissues, including kidney, liver, heart, muscle, and gonad. While the metabolic kinetics of sodium selenite in plasma, liver, and kidney of rats was well reflected by a two-compartment open model, that in heart and gonad was fitted to a one-compartment open model, and that in muscle was fitted to a one-compartment open model with a lag time. The results indicate that sodium selenite was absorbed by plasma and tissues quickly and was eliminated slowly after intragastric administration. Based on the results, we propose that multi-supplementation of Se with low dosage is superior to single supplementation with high dosage, in terms of avoiding selenosis.



Similar content being viewed by others
References
EFSA (2016) Safety and efficacy of selenium compounds (E8) as feed additives for all animal species: sodium selenite, based on a dossier submitted by Todini and Co SpA. EFSA Journal 14:4442
Zduńczyk Z, Drażbo A, Juśkiewicz J, Juskiewicz J, Czech A, Antoszkiewicz Z (2013) The effect of different dietary levels of vitamin E and selenium on antioxidant status and immunological markers in serum of laying hens. Pol J Vet Sci 16:333–339
Reid ME, Duffield-Lillico AJ, Sunga A, Fakih M, Alberts DS, Marshall JR (2006) Selenium supplementation and colorectal adenomas: an analysis of the nutritional prevention of cancer trial. Int J Cancer 118:1777–1781
Reid ME, Duffield-Lillico AJ, Garland L, Turnbull BW, Clark LC, Marshall JR (2002) Selenium supplementation and lung cancer incidence: an update of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev 11:1285–1291
Lemire M, Fillion M, Frenette B, Passos CJS, Guimarães JRD, Jr BF, Mergler D (2011) Selenium from dietary sources and motor functions in the Brazilian Amazon. Neurotoxicology 32:944–953
Styblo M, Thomas DJ (2001) Selenium modifies the metabolism and toxicity of arsenic in primary rat hepatocytes. Toxicol Appl Pharm 172:52–61
Davis TZ, Tiwary AK, Stegelmeier BL, Pfister JA, Panter KE, Hall JO (2017) Comparative oral dose toxicokinetics of sodium selenite and selenomethionine. J Appl Toxicol 37:231–238
Panter KE, Hartley WJ, James LF, Mayland HF, Stegelmeier BL, Kechele PO (1996) Comparative toxicity of selenium from seleno-dl-methionine, sodium selenate, and Astragalus bisulcatus in pigs. Fundam Appl Toxicol 32:217–223
Vinceti M, Mandrioli J, Borella P, Michalke B, Tsatsakis A, Finkelstein Y (2014) Selenium neurotoxicity in humans: bridging laboratory and epidemiologic studies. Toxicol Lett 230:295–303
Aldosary BM, Sutter ME, Schwartz M, Morgan BW (2012) Case series of selenium toxicity from a nutritional supplement. Clin Toxicol 50:57–64
Sutter ME, Thomas JD, Brown J, Morgan B (2008) Selenium toxicity: a case of selenosis caused by a nutritional supplement. Ann Intern Med 148:970–971
Seker FB, Sibel A, Baria O (2008) Lifelong consumption of sodium selenite: gender differences on blood–brain barrier permeability in convulsive, hypoglycemic rats. Biol Trace Elem Res 124:12–19
Ayaz M, Dalkilic N, Bariskaner H, Tuncer S, Demirel I (2007) Gender-dependent effects of selenite on the perfused rat heart. Biol Trace Elem Res 116:301–310
Minami T, Sakita Y, Ichida S, Dohi Y (2002) Gender difference regarding selenium penetration into the mouse brain. Biol Trace Elem Res 89:85–93
Zheng S, Xing H, Zhang Q, Xue H, Zhu F, Xu S (2019) Pharmacokinetics of sodium selenite administered orally in blood and tissues of selenium-deficient ducklings. Biol Trace Elem Res 190:509–516
Brodin O, Eksborg S, Wallenberg M, Asker-Hagelberg C, Larsen EH, Mohlkert D, Lenneby-Helleday C, Jacobsson H, Linder S, Misra S, Bjornstedt M (2015) Pharmacokinetics and toxicity of sodium selenite in the treatment of patients with carcinoma in a phase I clinical trial: the SECAR study. Nutrients 7:4978–4994
Fu L, Shi SY (2019) A novel strategy to determine the compositions of inorganic elements in fruit wines using ICP-MS/MS. Food Chem 299:125–172
Kwon Y (2002) Handbook of essential pharmacokinetics, pharmacodynamics and drug metabolism for industrial scientists, Kluwer Academic Publishers, pp 8–28
Orct T, Jurasovic J, Micek V, Karaica D, Sabolic I (2017) Macro- and microelements in the rat liver, kidneys, and brain tissues; sex differences and effect of blood removal by perfusion in vivo. J Trace Elem Med Biol 40:104–111
Finley JW, Kincaid RL (1991) Effect of sex and time of sampling on selenium and glutathione peroxidase activity in tissues of mature rats. Biol Trace Elem Res 29:181–191
Loeschner K, Hadrup N, Hansen M, Pereira SA, Gammelgaard B, Moller LH, Mortensen A, Lam HR, Larsen EH (2014) Absorption, distribution, metabolism and excretion of selenium following oral administration of elemental selenium nanoparticles or selenite in rats. Metallomics 6:330–337
Bates JM, Spate VL, Morris JS, St Germain DL, Galton VA (2000) Effects of selenium deficiency on tissue selenium content, deiodinase activity, and thyroid hormone economy in the rat during development. Endocrinology 141:2490–2500
Finley JW (2006) Bioavailability of selenium from foods. Nutr Rev 64:146–151
Whanger PD, Pedersen ND, Hatfield J, Weswig PH (1976) Absorption of selenite and selenomethionine from ligated digestive tract segments in rats. Proc Soc Exp Biol Med 153:295–297
Bunglavan SJ, Garg AK, Dass RS, Shrivastava S (2018) Effect of varied levels of selenium supplementation in nano form on growth, nutrient intake and digestibility in Wistar albino rats. Indian J Anim Res 52:248–253
Pedrero Z, Murillo S, Cámara C, Schram E, Luten JB, Feldmann I, Jakubowskie N, Madrid Y (2011) Selenium speciation in different organs of African catfish (Clarias gariepinus) enriched through a selenium-enriched garlic based diet. J Anal At Spectrom 26:116–125
Vindry C, Ohlmann T, Chavatte L (2018) Selenium metabolism, regulation, and sex differences in mammals. In: Michalke B (ed) Selenium. Springer International Publishing, Cham, pp 89–107
Suzuki Y, Hashiura Y, Matsumura K, Matsukawa T, Shinohara A, Furuta N (2010) Dynamic pathways of selenium metabolism and excretion in mice under different selenium nutritional statuses. Metallomics 2:126–132
Suzuki KT, Doi C, Suzuki N (2006) Metabolism of 76Se-methylselenocysteine compared with that of 77Se-selenomethionine and 82Se-selenite. Toxicol Appl Pharmacol 217:185–195
Nemoto M (2003) Experimental evaluation of the influence of complete artificial circulation on renal circulation and tissue metabolism—comparative study of pulsatile vs nonpulsatile circulation. Ann Thorac Cardiovasc Surg 9:355–364
NCI, DCPC Chemoprevention Branch and Agent Development Committee (1996) Clinical development plan: l-selenomethionine. J Cell Biochem Suppl 26:202–218
Waters DJ, Chiang EC, Cooley DM, Morris JS (2004) Making sense of sex and supplements: differences in the anticarcinogenic effects of selenium in men and women. Mutat Res 551:91–107
Behne D, Hofer T, von Berswordt-Wallrabe R, Elger W (1982) Selenium in the testis of the rat: studies on its regulation and its importance for the organism. J Nutr 112:1682–1687
Hill KE, Zhou J, Austin LM, Motley AK, Ham A-JL, Olson GE, Atkins JF, Gesteland RF, Burk RF (2007) The selenium-rich C-terminal domain of mouse selenoprotein P is necessary for the supply of selenium to brain and testis but not for the maintenance of whole body selenium. J Biol Chem 282:10972–10980
Stahl W, Berg Hvd, Arthur J, Bast A, Dainty J, Faulks RM, Gartner C, Haenen G, Hollman P, Holst B, Kelly FJ, Polidori MC, Rice-Evans C, Southon S, Vliet Tv, Vina-Ribes J, Williamson G, Astley SB (2002) Bioavailability and metabolism. Mol Asp Med 23:39–100
Siscar R, Koenig S, Torreblanca A, Solé M (2014) The role of metallothionein and selenium in metal detoxification in the liver of deep-sea fish from the NW Mediterranean Sea. Sci Total Environ 466-467:898–905
Lailsonbrito J, Cruz R, Dorneles PR, Andrade L, Azevedo AF, Fragoso AB, Vidal LG, Costa MB, Bisi TL, Almeida R (2012) Mercury-selenium relationships in liver of Guiana dolphin: the possible role of Kupffer cells in the detoxification process by tiemannite formation. PLoS One 7:e42162
García-Sevillano MA, Rodríguez-Moro G, García-Barrera T, Navarro F, Gómez-Ariza JL (2015) Biological interactions between mercury and selenium in distribution and detoxification processes in mice under controlled exposure. Effects on selenoprotein. Chem Biol Interact 229:82–90
Ikemoto T, Kunito T, Tanaka H, Baba N, Miyazaki N, Tanabe S (2004) Detoxification mechanism of heavy metals in marine mammals and seabirds: interaction of selenium with mercury, silver, copper, zinc, and cadmium in liver. Arch Environ Contam Toxicol 47:402–413
Yang J, Kunito T, Tanabe S, Miyazaki N (2007) Mercury and its relation with selenium in the liver of Dall’s porpoises (Phocoenoides dalli) off the Sanriku coast of Japan. Environ Pollut 148:669–673
Suzuki KT, Tsuji Y, Ohta Y, Suzuki N (2008) Preferential organ distribution of methylselenol source Se-methylselenocysteine relative to methylseleninic acid. Toxicol Appl Pharmacol 227:76–83
Steinbrenner H, Sies H (2009) Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta 1790:1478–1485
Van Der Torre HW, Dokkum W, Van Schaafsma G, Wedel M, Ockhuizen T (1991) Effect of various levels of selenium in wheat and meat on blood Se status indices and on Se balance in Dutch men. Br J Nutr 65:69–80
Sun K, Wu S, Wang Y, Wan X, Thompson HJ, Zhang J (2013) High-dose sodium selenite toxicity cannot be prevented by the co-administration of pharmacological levels of epigallocatechin-3-gallate which in turn aggravates the toxicity. Food Chem Toxicol 52:36–41
Reid ME, Stratton MS, Lillico AJ, Fakih M, Natarajan R, Clark LC, Marshall JR (2004) A report of high-dose selenium supplementation: response and toxicities. J Trace Elem Med Biol 18:69–74
Acknowledgments
We thank Prof. Guoping Yan (Wuhan Institute of Technology) for the helpful suggestion in the revision of the manuscript.
Funding
This work was financially supported by the Natural Science Foundation of Hubei Province (No. 2016CFB212), Major Project of Technical Innovation in Hubei Province (No. 2017ABA156), Hubei Provincial Health and Family Planning Commission (No. WJ2019Q054), and National Natural Science Foundation of China (No. 51703173).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 247 kb)
Rights and permissions
About this article
Cite this article
Zeng, X., Zhang, X., Fan, B. et al. Pharmacokinetics of Sodium Selenite in Rat Plasma and Tissues After Intragastric Administration. Biol Trace Elem Res 196, 494–501 (2020). https://doi.org/10.1007/s12011-019-01928-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01928-8