Abstracts
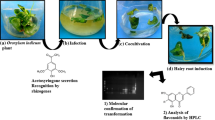
Catharanthus roseus (L.) G. Don is a plant species known for its production of a variety of terpenoid indole alkaloids, many of which have pharmacological activities. Catharanthine can be chemically coupled to the abundant leaf alkaloid vindoline to form the valuable anticancer drug vinblastine. To study and extract catharanthine and other metabolites from C. roseus, a technique was developed for producing hairy root cultures. In this study, the Agrobacterium rhizogenes A4 was induced in the hairy roots from leaf explants, and the concentration of antibiotics (100 mg/L kanamycin) was elucidated for selection after transformation. The polymerase chain reaction amplification of rol genes results revealed that transgenic hairy roots contained rol genes from the root induced (Ri)-plasmid. Catharanthine from C. roseus hairy roots was separated and analyzed using high-performance liquid chromatography. Over-expression of CrOrca3 (octadecanoid-responsive Catharanthus AP2/ERF domain), and cytohistochemical staining methods were used to validate transgenic hairy roots from C. roseus. Hairy root culture of C. roseus is a valuable approach for future efforts in the metabolic engineering of terpenoid indole alkaloids in plants.





Similar content being viewed by others
References
Alpizar, E., Dechamp, E., Espeout, S., Royer, M., Lecouls, A. C., Nicole, M., et al. (2006). Efficient production of Agrobacterium rhizogenes-transformed roots and composite plants for studying gene expression in coffee roots. Plant Cell Reports, 25, 959–967.
Aoki, T., Matsumoto, H., Asako, Y., Matsunaga, Y., & Shimomura, K. (1997). Variation of alkaloid productivity among several clones of hairy roots and regenerated plants of Atropa belladonna transformed with Agrobacterium rhizogenes 15834. Plant Cell Reports, 16, 282–286.
Batra, J., Dutta, A., Singh, D., Kumar, S., & Sen, J. (2004). Growth and terpenoid indole alkaloid production in Catharanthus roseus hairy root clones in relation to left- and right-termini-linked Ri T-DNA gene integration. Plant Cell Reports, 23, 148–154.
Christey, M. C. (2001). Use of Ri-mediated transformation for production of transgenic plants. In Vitro and Cellular Developmental Biology-Plant, 37, 687–700.
Ciau-Uitz, R., Miranda-Ham, M. L., Coello-Coello, J., Chi, B., Pacheco, L. M., & Loyola-Vargas, V. M. (1994). Indole alkaloid production by transformed and non-transformed root cultures of Catharanthus roseus. In Vitro Cellular & Developmental Biology, 30, 84–88.
Gamborg, O. L., Miller, R. A., & Ojima, K. (1968). Nutrient requirements of suspension cultures of soybean root cells. Experimental Cell Research, 50, 151–158.
Jefferson, R. (1987). Assaying chimeric genes in plants: the GUS gene fusion system. Plant Molecular Biology Reporter, 5, 387–405.
Jouanin, L. (1984). Restriction map of an agropine-type Ri plasmid and its homologies with Ti plasmids. Plasmid, 12, 91–102.
Jouhikainen, K., Lindgren, L., Jokelainen, T., Hiltunen, R., Teeri, T. H., & Oksman-Caldentey, K. M. (1999). Enhancement of scopolamine production in Hyoscyamus muticus L. hairy root cultures by genetic engineering. Planta, 208, 545–551.
Kim, Y. J., Wyslouzil, B. E., & Weathers, P. J. (2002). Secondary metabolism of hairy root cultures in bioreactors. In Vitro and Cellular Developmental Biology-Plant, 38, 1–10.
Kohli, A., Twyman, R. M., Abranches, R., Wegel, E., Stoger, E., & Christou, P. (2003). Transgene integration, organization and interaction in plants. Plant Molecular Biology, 52, 247–258.
Kumar, V., Satyanarayana, K. V., Itty, S. S., Indu, E. P., Giridhar, P., Chandrashekar, A., et al. (2005). Stable transformation and direct regeneration in Coffea canephora P ex. Fr. by Agrobacterium rhizogenes mediated transformation without hairy-root phenotype. Plant Cell Reports, 25, 214–222.
Menke, F. L. H., Champion, A., Kijne, J. W., & Memelink, J. (1999). A novel jasmonate- and elicitor-responsive element in the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate- and elicitor-inducible AP2-domain transcription factor, ORCA2. EMBO Journal, 18, 4455–4463.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum, 15, 473–497.
Park, N. I., Tuan, P. A., Li, X. H., Kim, Y. K., Yang, T. J., & Park, S. U. (2011). An efficient protocol for genetic transformation of Platycodon grandiflorum with Agrobacterium rhizogenes. Molecular Biology Reports, 38, 2307–2313.
Peebles, C. A. M., Erik, H. H., Jacqueline, V. S., & San, K. Y. (2009). Transcriptional response of the terpenoid indole alkaloid pathway to the overexpression of ORCA3 along with jasmonic acid elicitation of Catharanthus roseus hairy roots over time. Metabolic Engineering, 11, 76–86.
Shahin, E. A., Sukhapinda, K., Simpson, R. B., & Spivey, R. (1986). Transformation of cultivated tomato by a binary vector in Agrobacterium rhizogenes: transgenic plants with normal phenotypes harbour binary vector T-DNA, but no Ri-plasmid T-DNA. Theoretical and Applied Genetics, 72, 770–777.
Shalel-Levanon, S., San, K. Y., & Bennett, G. N. (2005). Effect of oxygen, and ArcA and FNR regulators on the expression of genes related to the electron transfer chain and the TCA cycle in Escherichia coli. Metabolic Engineering, 7, 364–374.
Stewart, C. N., Jr., & Via, L. E. (1993). A rapid CTAB DNA isolation technique for RAPD finger print and other PCR applications. Biotechniques, 14, 748–750.
Tikhomiroff, C., & Jolicoeur, M. (2002). Screening of Catharanthus roseus secondary metabolites by high-performance liquid chromatography. Journal of Chromatography. A, 955, 87–93.
Van der Fits, L., & Memelink, J. (2000). ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science, 289, 295–297.
White, F. F., Taylor, B. H., Huffman, G. A., Gordon, M. P., & Nester, E. W. (1985). Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of Agrobacterium rhizogenes. Journal of Bacteriology, 164, 33–44.
Zhou, M. L., Shao, J. R., & Tang, Y. X. (2009). Production and metabolic engineering of terpenoid indole alkaloids in cell cultures of the medicinal plant Catharanthus roseus (L.) G. Don (Madagascar periwinkle). Biotechnology and Applied Biochemistry, 52, 313–323.
Zhou, M. L., Zhu, X. M., Shao, J. R., Wu, Y. M., & Tang, Y. X. (2010). Transcriptional response of the catharanthine biosynthesis pathway to methyl jasmonate/nitric oxide elicitation in Catharanthus roseus hairy root culture. Applied Microbiology and Biotechnology, 88, 737–750.
Zhou, M. L., Zhu, X. M., Shao, J. R., Tang, Y. X., & Wu, Y. M. (2011). Production and metabolic engineering of bioactive substances in plant hairy root culture. Applied Microbiology and Biotechnology, 90, 1229–1239.
Zhou, M. L., Hou, H. L., Zhu, X. M., Shao, J. R., Wu, Y. M., & Tang, Y. X. (2011). Soybean transcription factor GmMYBZ2 represses catharanthine biosynthesis in hairy roots of Catharanthus roseus. Applied Microbiology and Biotechnology, 91, 1095–1105.
Acknowledgments
We thank Pang Jun-Feng for technical assistance with RNA extraction and Du Hai for work discussions. We are also grateful to Trillian Ross (Plant Cell Physiology, Institute of Biology, Leiden University, The Netherlands) for giving some useful comments on this manuscript. This work was supported by a grant from the National Transgenic Program (2009ZX08005-004B and 2009ZX08009055B).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhou, ML., Zhu, XM., Shao, JR. et al. An Protocol for Genetic Transformation of Catharanthus roseus by Agrobacterium rhizogenes A4. Appl Biochem Biotechnol 166, 1674–1684 (2012). https://doi.org/10.1007/s12010-012-9568-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9568-0