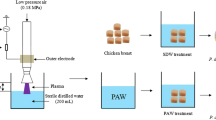
Abstract
Ensuring food safety measures are essential to minimize the risk of foodborne diseases linked to raw food products. Here, we investigated the efficacy of an innovative approach for the control of Salmonella Typhimurium found in fresh produces. Plasma activated water (PAW) and bacteriophages are emerging effective and valuable alternative methods for microbiological decontamination. The efficacy of PAW and a lytic bacteriophage (109 PFU/mL), both separately and sequentially, against S. Typhimurium in fresh produce was investigated. S. Typhimurium (105–107 CFU/g) were inoculated on lettuce leaves and treated with PAW, S. Typhimurium phage SK-T2 or their combination. PAW or bacteriophage inactivated S. Typhimurium, on lettuce leaves at different initial populations, by 2.90–3.46 or 1.45–3.25 log CFU/g, respectively. After sequential treatments of PAW and bacteriophage, S. Typhimurium populations, initially applied at ~ 105 CFU/g reduced by 4.47 log CFU/g, but when the order of application was changed (i.e., bacteriophage followed by PAW), the combination synergistically decreased the Salmonella numbers below the detection limit of the method used for the enumeration (i.e., < 101 CFU/g). At the high-level inoculum (~ 7 log CFU/g), consecutive treatments of PAW and phage decreased the S. Typhimurium population by 3.28 log CFU/g, and a reduction of 6.20 log CFU/g was achieved after reversing the order of treatment. Regardless of the bacterial inoculum level, sequential applications of bacteriophage and PAW resulted in a higher level of inactivation. This study proved that the bacteriophage–PAW combination constitutes a promising alternative approach to the conventional washing process in fresh produce wash waters in the food industry.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increase in the demand for fresh-cut food produce reflects changing consumer preferences and the growing emphasis on convenience and health-conscious eating habits in modern societies. Accordingly, the rise in foodborne outbreaks from raw produce is a concerning trend and has raised awareness of the cruciality of food safety and proper handling of fresh vegetables and fruits. Fresh or freshly cut fruit and vegetables can be potential carriers of foodborne microorganisms due to their high nutrient and water content (Han et al., 2022; Pan et al., 2019), as well as their exposure to contaminants such as sewage water during the production process (Luna-Guevara et al., 2019). Salmonella contamination through food usually originates from poultry and poultry products (Evran et al., 2022; Williams et al., 2020). However, in addition to Listeria monocytogenes, Shiga toxin-producing Escherichia coli and Salmonella contamination and diseases are also known to arise from fresh fruits and vegetables (Shang et al., 2023; Bartz et al., 2017; Bennett et al., 2015, 2018; Hou et al., 2021)). Addressing food safety issues related to fresh or minimally processed produces is essential, but consumer concerns, energy consumption, and toxicity risks also need to be carefully considered. Today, the most common method used to decontaminate pathogenic microorganisms in fresh produce is washing the produce with chlorine-based disinfectants such as chlorine gas, sodium hypochlorite, chlorine dioxide, and calcium hypochlorite. These chlorine-based disinfectants can reduce the microbial load on the product by approximately 1–2 logs, in which case the intended decontamination effect is not achieved (Gragg & Brashears, 2010; Vengarai Jagannathan et al., 2021). It is known that the use of chemical disinfectants at high concentrations raises significant concerns about the possibilities of by-product formation and its effects on health and the environment. (Gil et al., 2009; Patange et al., 2019; Van Haute et al., 2013). In addition, many pathogens have become resistant to traditional sanitizing agents such as quaternary ammonium compounds and chlorine, which are used to ensure the microbial safety of products and provide a more stable and long shelf life (Verraes et al., 2013). New residue-free chemical intervention technologies with effective antimicrobial activity are essential to ensure microbial safety without undesirable impacts on organoleptic properties of fresh products.
Recently, cold plasma gained attention because of its application as a new novel non-thermal technology in the food industry. Plasma is known as the fourth state of matter and is a partially ionized gas composed of reactive chemical and physical species such as UV, electrons, ions, and free radicals (Han et al., 2022; Pankaj & Keener, 2017). Gas molecules excited by electrical discharges are used to create plasma. Exposure of water or its surface to cold plasma discharge results in the transfer of reactive plasma species to the water environment. This process brings about changes in the properties and chemistry of water, leading to the formation of PAW (Shah et al., 2023; Thirumdas et al., 2018). Particles generated by plasma meet water molecules resulting in different types of reagents that exhibit important biological activities in biomedical and agricultural applications. Various chemical reactions that may occur lead to acidification of water with the emergence of reactive oxygen and nitrogen species (RONS) during the formation of PAW (Shah et al., 2023; Thirumdas et al., 2018). PAW generates reactive species, including short-lived species like superoxide, singlet oxygen, hydroxyl radical, nitric oxide, peroxynitrite, and peroxynitric and long-lived species such as nitrate, nitrite, ozone, and hydrogen peroxide. The composition and amount of reactive species present in PAW are influenced by production techniques, including input voltage, plasma activation, storage duration, and the source of water (Han et al., 2022; Lim et al., 2021; Lin et al., 2019; Liu et al., 2020; Ma et al., 2016; Risa Vaka et al., 2019).
In addition to biomedical applications, PAW has been widely investigated in the food and agricultural industry (Kaushik et al., 2019; Shah et al., 2023). There are several studies showing that PAW can be used to eliminate both pathogenic and spoilage microorganisms in food products such as mushrooms (Agun et al., 2021; Hou et al., 2021), strawberries (Hou et al., 2021; Misra et al., 2014), bean sprouts (Hou et al., 2021; Xiang et al., 2018), Korean rice cake (Han et al., 2020; Hou et al., 2021), eggs (Hou et al., 2021; Lin et al., 2019), and cabbage (Choi et al., 2019; Hou et al., 2021). PAW was found to be effective against Salmonella Typhimurium (Shah et al., 2023), Listeria monocytogenes and E. coli O157:H7 (Han et al., 2020), methicillin-resistant and susceptible Staphylococcus aureus (Han et al., 2020; Shah et al., 2023; Wang et al., 2021), and natural microbiota in fresh beef (Shah et al., 2023; Zhao et al., 2020). Previous studies have represented that PAW is a promising approach in decontaminating produce without causing unwanted defects (Cong et al., 2022; Gavahian et al., 2020; Guo et al., 2017; Han et al., 2022; Lim et al., 2021; Ma et al., 2015; Zhao et al., 2019).
Different non-thermal methods have been studied in combination with PAW to improve its decontamination efficiency in the food industry. While the synergistic interaction of PAW with mild heat (Choi et al., 2019; Han et al., 2022), blanching (Han et al., 2022; Muhammad et al., 2019), and ultrasound (Han et al., 2022; Zhao et al., 2021) has been studied, no study has yet been conducted to examine its effect in combination with bacteriophages.
Bacteriophages, commonly referred to as phages, are viral entities that specifically target prokaryotic cells (Choińska-Pulit et al., 2015). Phages are characterized as entities with remarkable efficacy in targeting the most common bacterial species in our ecosystem. Their estimated abundance ranges from 1030 to 1032, exceeding their host bacterial counterparts tenfold (Marcó et al., 2012). Following their discovery, it has been investigated in various laboratory and clinical trials that phages may be an alternative strategy in combating bacterial infections. Although their importance declined with the advent of antibiotics, especially in Western medicine, recent years have witnessed a resurgence in their importance due to the greatly increasing problem of antibiotic resistance (Chanishvili, 2012; Gordillo Altamirano & Barr, 2019). Apart from their application in human phage therapy, phages find usage in different sectors, including food production, agriculture, and veterinary medicine. Their role extends to enhancing food safety through applications such as reducing microbial populations in livestock, managing microbial loads during food processing, ensuring the sanitation of surfaces and equipment that interact with food, and protecting end products against contamination during storage (Sillankorva et al., 2012). In the literature, the effectiveness of phages against Salmonella spp. has been studied in many foods ranging from milk to cheese, ready-to-eat foods, and meat products (Guo et al., 2021; Islam et al., 2019; Modi et al., 2001; Phongtang et al., 2019; Zinno et al., 2014). Phages can be safely applied on fresh foods in a wide range of doses. For example, there is a commercial phage preparation called Listex™ P100, approved by the FDA, which contains 1011 PFU phage per ml and can be used against Listeria spp. in foods (BIOHAZ, 2016). Similarly, in another study using high titers of Salmonella spp. phages, it was found that there was no adverse change in the sensory properties of the food groups tested, including lettuce (Guo et al., 2021). All these suggest that phages have a significant potential for the use as biocontrol agents in fresh foods.
The aim of this study was to evaluate the synergistic effect of two innovative non-thermal methods and to demonstrate their applicability in fresh foods. For this purpose, PAW and phage were used both separately and sequentially in the present study to assess the inhibition effect on Salmonella. Salmonella Typhimurium were inoculated onto lettuce leaves at two different initial concentrations and then counted when PAW and phage treatments were applied both separately and sequentially. It is believed that this gap in the literature will be addressed, as there is no study examining the combined or sequential use of these two known effective sanitation methods.
Materials and Methods
Bacterial Strains, Bacteriophage, and Growth Media
Salmonella Typhimurium ATCC BAA-190 (S. Typhimurium) was previously used in a study by (Soykut et al., 2019) and kindly obtained from Prof. Dr. Sami Fattouch. The S. Typhimurium phage SK-T2 used in the present study was isolated and characterized in a previous study (Evran et al., 2022).
Agar and Tryptic Soy Broth used for preparation of bacterial and bacteriophage stocks were obtained from Merck (Merck Millipore Corporation, Darmstadt, Germany). Glycerol (Merck Millipore Corporation) at 50% (v/v) was used to prepare the stocks of bacteria and phages and all stocks were stored at − 18 °C during the study. Tryptic Soy Broth (Merck Millipore Corporation), soft agar (0.6%), and agar (1.5%) were used for phage titer. DNase and RNase (Sigma-Aldrich, St. Louis, MO) were used for phage purification (Tayyarcan et al., 2022). Concentrated phages were resuspended in phosphate-buffered saline (PBS). Peptone water (PW) and Xylose Lysine Deoxycholate (XLD) agar were used for the enumeration of Salmonella and were provided from Merck Millipore (Darmstadt, Germany).
Bacteria Enumeration
S. Typhimurium was counted before and after of PAW, phage, and sequential treatments. Briefly, treated and control group lettuce leaves were placed into 20 mL PW and shaken at 140 rpm for 30 min. After that, 1 mL of samples was taken into sterile Eppendorf tubes and serial dilutions were prepared. Serial dilutions were spread onto XLD agar and incubated at 37 °C for 18–24 h. Following the incubation, typical S. Typhimurium colonies were counted.
PAW Preparation and Conditions
Figure 1 represents the schematic of the atmospheric cold plasma (ACP) system used in the present study. The PAW was prepared using Openair® plasma system (Plasmatreat GmbH, Steinhagen, Germany) which includes a generator (AS400FG), a plasma jet with a rotation adaptor (PFW10-RV1004), a transformer (HTR11), pressure supply unit (DVE20), and gas connectors described in our previous studies (Dasan & Boyaci, 2018). The plasma was created by discharging at high voltage using a single electrode, generating a plasma discharge that exited at high velocity from the jet nozzle to the water surface. Additionally, the utilization of a dual nozzle system comprising of two distinct plasma nozzles revolving jointly around a rotary axis was employed to guarantee a notably intense and uniform treatment. This revolving nozzle grants larger magnitudes to be exposed to equalized plasma treatment with a diminished temperature surge in the treated zone. As a feed gas, compressed and filtered dry air at room temperature (flow rate of 50 L/min) was used. The ACP system was operated by using 650 W power for 10 min. PAW was generated by exposing 200 mL of deionized water (DI) in a cylindrical borosilicate glass beaker with a total volume of 600 mL to this plasma and the plasma is evenly distributed over the water by rotating the nozzle. Such a plasma source produces a three-dimensional cone-shaped plasma jet rotating around its axis extending out of a 15–30 mm diameter capillary tube about 30 mm long at an air gas flow of 50 L/min.
After the PAW formation was completed, pH measurement was taken when the PAW temperature reached 25 °C. The pH value of PAW was determined as 2.8.
Storage conditions are critical to prolong the antimicrobial activity of PAW. A decreased inactivation efficacy due to the post-production storage of PAW has been observed (Smet et al., 2019). Therefore, immediately after PAW production, the final temperature was always below 45 °C and experiments were conducted after the PAW was kept at 4 °C for one hour after production.
Bacteriophage Preparation
Lytic bacteriophage of S. Typhimurium phage SK-T2 was used for phage treatments. The phage solution for use in all phage treatments was concentrated and purified by the following experimental steps. Host bacterium and phage were inoculated in 100 mL broth and left to incubate at 37 °C for 5 h with shaking to achieve a final MOI of 0.01. Following the incubation, the phage and bacterial mixture was centrifuged at 12 000 × g for 6 min at 4 °C. Then, the phage supernatant was passed through of a 0.22-µm syringe filter (Sartorius, Göttingen, Germany). Before phage purification, the titers of phages were determined using the double layer agar method. For phage supernatant purification, NaCl was added to a final concentration of 1 M, and incubation was performed in an ice bath at 4 °C for 1 h. After incubation, centrifugation was performed at 18 000 × g for 15 min at 4 °C. Then, PEG 8000 was added at 10% of the volume of the phage supernatant and gently mixed until the peg was completely dissolved. The mixture was incubated in an ice bath at 4 °C for 18–24 h and then centrifuged at 18 000 × g for 15 min at 4 °C. After the centrifugation step, the supernatant was removed, and the pellet was resuspended with PBS buffer. The purified and concentrated phages were stored at 4 °C for the experiments (Ekiz et al., 2023).
Inoculation of Lettuce Leaves
Lettuce was obtained from the local market from Ankara, Turkiye and stored at 4 °C until used in the experiments. Lettuce leaves were cut into 1 g each and then soaked in 70% ethanol for 5 min after washing with tap water to remove visual debris. The cut lettuce leaves were immersed in sterile deionized water and placed individually in a sterile petri dish. 100 µL of S. Typhimurium bacterial solution (106 CFU/mL for 105 CFU/gr; 108 CFU/mL for 107 CFU/gr) was inoculated onto lettuce leaves using the spot inoculation method, with final concentrations of approximately 105 (lower microbial load, LML) and 107 (higher microbial load, HML) CFU per gram. Lettuce leaves were kept at room temperature for 6 h, so that the inoculated bacteria would adhere to the lettuce surface and dry before treatments.
PAW Application on Lettuce
The previously prepared PAW solution was divided into 50 mL each and used as wash water. One g of sample in triplicate was transferred to 50 mL of PAW solution and shaken at 110 rpm for 10 min to simulate washing conditions. Bacterial count was detected the method that described at bacterial enumeration section.
Bacteriophage Application on Lettuce
50 mL of deionized water was used as wash water for phage treatments. Purified phage was added to 50 mL of deionized water to adjust the final concentration to 109 PFU/mL. Three parallel treatment samples were washed with phage-added deionized water by shaking at 110 rpm for 10 min. Then, lettuce samples were incubated at room temperature for 2 h. Bacterial count was detected the method that described previously.
Sequential Treatment with PAW Followed by Bacteriophage
Three parallel 1-g samples were placed in 50 mL PAW solution with shaking at 110 rpm for 10 min before sequential treatment with phage. Immediately after PAW treatment, the samples were transferred to 50 mL of phage solution and allowed to stand for 10 min with shaking at 110 rpm and the count of bacteria was determined.
Sequential Treatment with Bacteriophage Followed by PAW
For the treatment, samples were transferred to 50 mL of phage solution (~ 109 PFU/mL phage) at 110 rpm for 10 min with continuous shaking. After washing, the samples were held at room temperature for two hours. Then, the samples were dropped into 50 mL PAW solution with continuous shaking at 110 rpm for 10 min; then the bacteria were enumerated.
Statistical Analysis
The data in this study were presented as mean of three independent experiments, and the error bars show the standard deviations. Fisher’s least significant difference one-way ANOVA (Minitab 17.1.1, Minitab LLC, UK) was used to examine significant differences (p < 0.05) between the sample groups.
Result and Discussion
Effect of PAW Treatment on Bacterial Count
PAW alone was used for bacterial decontamination on lettuce. Lettuce leaves loaded with two different concentrations of S. Typhimurium (~ 105 and 107 CFU/g) were washed with PAW for 10 min and immediately afterwards evaluated for bacterial counts. The bacterial load in the samples used as control and not washed with PAW was 5.71 and 7.18 log CFU/g, respectively. After 10 min of PAW treatment, bacterial counts of 2.25 and 4.28 log CFU/g were obtained for low and high bacterial load, respectively. Overall, the bacterial count was decreased by 3.46 and 2.90 log CFU/g for the low and high load samples, respectively (Fig. 2). As a self-control, washing the S. Typhimurium inoculated lettuce with sterile deionized water (not activated with plasma) did not cause any reduction resulted after 10 min. The difference in bacterial count between the control and treated samples was significant according to statistical analysis.
Bacterial counts on lettuce leaves inoculated with low (LML) and high (HML) numbers of bacteria when PAW was used as washing solution. Bacteria counts are presented for untreated (UT) and treated (T) samples. Control groups for both low and high inoculation were not treated with PAW. Bars were used for representing of standard error of mean. * indicates significant differences between samples (p < 0.05)
The inactivation efficiency of PAW depends on the concentration and composition of RONS, which is influenced by the plasma source and discharge and plasma operating parameters. Various plasma sources, such as gliding arc discharge, DBD, and plasma jet, can lead to varying levels of RONS production in the air plasma. This, in turn, affects their transportation into the liquid and the subsequent efficacy of PAW inactivation (Machala et al., 2018). Plasma jets and DBDs are generally the most widely used sources of plasma generation for PAWs, owing to their ability to produce reactive species and their simple structures. Long-lived RONS and short-lived RONS play significant roles in the inactivation process. Recent studies suggested that PAW had damaging effects on the cell membrane, integrity, and intracellular contents such as nucleic acids (Lin et al., 2020; Ma et al., 2020). As expected, the decontamination efficiency of the PAW treatment decreased as the initial bacterial load increased. A higher initial microbial load leads to an accumulation of cells on the surface of the sample. Stacking tends to reduce the efficiency of plasma inactivation as the enclosed microorganisms are shielded from the produced plasma species (Dasan et al., 2016).
Several studies indicated that PAW has strong antimicrobial activity on different types of microorganisms and can be used as disinfectant agent for fresh cut produce like spinach and kale. In the case of kale, a reduction of 3.48 log CFU/g was obtained, while in the case of spinach, complete inactivation (~ 6 log CFU/g) of E. coli population was observed in a 10 min of PAW (produced by 30 min plasma discharge) treatment (Perinban et al., 2022). Similarly, P. fluorescens and L. innocua on lettuce were reduced by 4.5 and 2.4 log CFU/g, respectively, after 5 min of PAW treatment (Patange et. al, 2019). In another study, Salmonella Enteritidis was reported to decrease from 7.92 log CFU/egg to 2.84 CFU/egg after 60 s of PAW (produced by 20 min plasma discharge) treatment (Lin et al., 2020).
Effect of Bacteriophage Treatment on Bacterial Count
The effect of phage on lettuce leaves loaded with two different bacterial concentrations (~ 105 and 107 CFU/g) was evaluated with using phage loaded washing water. The final phage concentration of the wash water was adjusted to 109 PFU/mL and used to wash the bacteria loaded lettuce leaves. Bacterial reduction was assessed after phage treatment. Washing with the phage solution decreased the bacterial numbers by 3.25 and 1.45 log CFU/g for 105 and 107 CFU/g loading, respectively (Fig. 3).
Bacterial counts on lettuce leaves inoculated with low (LML) and high (HML) numbers of bacteria when phage was used as washing solution. Bacteria counts are presented for untreated (UT) and treated (T) samples. Control groups for both LML and HML were not treated. Bars were used for representing of standard error of mean. * indicates significant differences between samples (p < 0.05)
Numerous studies conducted by different researchers have proven that bacteriophages have great potential to control pathogenic bacteria in fresh cut produce. Some studies have shown that bacteriophages are an effective tool to eliminate of E. coli on peppers (Snyder et al., 2016); L. monocytogenes for apple, melon, and pear (Oliveira et al., 2014); Salmonella Enteritidis on fresh-cut melons and apples (Leverentz et al., 2001); E. coli O104:H4 in alfalfa sprouts and seeds; and L. monocytogenes in ready to eat meat and cantaloupes (Lone et al., 2016). Although the use of phage caused a significant bacterial reduction (1.45 log CFU/g) in samples with high bacterial inoculation, the reduction was less than in the samples with low inoculation. A parallel result was obtained in a study by Yesil et al. (2023) that a single application of E. coli phage decreased the count of E. coli by 1.5 log CFU/g in spinach.
Effect of Sequential Treatment with PAW Followed by Bacteriophage
After individual applications, inoculated lettuce leaves were first treated with PAW, then washed with phage loaded deionized water and inoculated at room temperature for 2 h. Control samples and three parallel treated samples were used for sequential experiments. The bacterial counts of control and treated samples can be seen in Fig. 4 for low and high bacterial load. The counts of control samples were 5.62 and 7.26 log CFU/g for low and high bacterial load, respectively. The total reductions after successive sequential treatments were 4.47 and 3.28 log CFU/g for low and high loaded samples, respectively.
Bacterial counts on lettuce inoculated with low (LML) and high (HML) numbers of bacteria when PAW treatment followed by phage application. Bacteria counts are presented for untreated (UT) and treated (T) samples. No treatment was applied to the control groups for both LML and HML. Error bars were used to represent the standard deviations. * indicates significant differences between samples (p < 0.05)
PAW, as a non-thermal technology, has also been investigated in combination with another treatment, bacteriophage, to increase the efficacy of inactivation. The synergistic inactivation effects using combined treatments were higher than individual treatments. PAW followed by phage application resulted in a better reduction of S. Typhimurium than PAW or bacteriophage alone, possibly because PAW damages the cell structures of bacteria and bacteriophage intensifies the disruption. Similar results were obtained with the combined use of PAW with airborne acoustic ultrasound, showing better antimicrobial effects on the inactivation of E. coli biofilms, possibly because of the better diffusion of RONS into the matrix after ultrasound-induced physical damage (Charoux et al., 2020). Furthermore, Choi et al., 2019 stated that the sequential application of washing for 10 min with PAW (produced by 120 min plasma discharge) followed by mild heat treatment at 60 °C reduced S. aureus and L. monocytogenes counts up to 3.7 and 3.4 log CFU/g, respectively.
Effect of Sequential Treatment with Bacteriophage Followed by PAW
The experiment was carried out by reversing the order of application to determine the effect of the order of application on bacteria. Sequential treatment with phage following PAW was evaluated for bacterial reduction on lettuce leaves. Control samples and three parallel treated samples were used for the sequential experiments. Treatment groups were washed with phage added deionized water for 10 min, then incubated for 2 h and treated with PAW for 10 min. After all treatments, bacterial counts were calculated, and the results are represented in Fig. 5. The bacterial counts of untreated control groups were 5.18 and 6.83 log CFU/g for low and high loaded samples, respectively. After phage and PAW treatments, respectively, the bacterial counts of the low loaded samples remained below the detection limit (< 101 CFU/g). As a result, the total reduction was about 5.18 log CFU/g for low loaded samples. The bacterial count of the control group was calculated as 6.83 log CFU/g for high loaded samples, and the total reduction after successive sequential treatments was 6.20 log CFU/g. For all bacterial loads, the differences between control and treated samples were considered significant according to statistical analysis.
Bacterial counts on lettuce inoculated with low (LML) and high (HML) numbers of bacteria when bacteriophage application followed by PAW treatment. Bacteria counts are presented for untreated (UT) and treated (T). No treatment was applied to the control groups for both LML and HML. Bars were used for representing of standard error of mean. * indicates significant differences between samples (p < 0.05)
Many studies have shown that bacteriophage and PAW treatments are separately effective in controlling pathogenic bacteria in fresh-cut foods. However, sequential treatments lead to a greater reduction in bacterial counts. Although phage and PAW alone reduced the bacterial load, the sequential treatments of phage and PAW resulted in a much greater reduction, especially in the group with the high initial load. Many studies in the literature have reported better effects when phage is administered sequentially or in combination with another antimicrobial (synergistic effect). In the study of Yesil et al. (2023), spinach leaves inoculated with two different bacterial loads (105 and 107 CFU/g) were subjected to phage and ozone treatments. The study found that the sequential application effectively reduced the bacterial population in spinach with an initial load of 105 CFU/g to levels below the detection limit. They also observed that sequential use of phage and ozone treatments in resulted in a greater reduction in bacterial loads than when each method was used separately (Yesil et al., 2023).
At low initial load, the sequential use of phage and PAW reduced the bacterial count below the detection limit. When the initial load was low initial loads, both the inhibition effect is expected to be greater than at high initial load and the count of bacteria is expected to decrease below the detection limit.
Among the four treatments (phage; PAW; PAW followed by phage; phage followed by PAW), the most significant reduction in bacterial numbers occurred when the phage was applied first, followed by the PAW treatment. A similar result was also obtained in a study by Stachler et al. (2021), who reported that phage treatment before chemical disinfectants inactivated Pseudomonas aeruginosa and had a better effect than the treatments alone. In contrast, when a chemical disinfectant was applied before phage treatment, the additional benefit of phage treatment was reported to become negligible (Stachler et al., 2021). Furthermore, the increase in decontamination efficiency when the sequence is reversed can be attributed to different approaches. Primarily, the phage acted on bacterial cells, reducing the bacterial load, and lowering it to a concentration where PAW could act faster and more easily. It is generally accepted that the inactivation ability of PAW is linked to the formation of several RONS. Therefore, the concentration of RONS to act on a unit bacterial cell may have increased. The reduction of bacteria was found to be significantly greater in the acidic environment of PAW due to its synergistic effects. On the other hand, a low pH condition had only a minimal impact (Patange et al., 2019) but could have effects on bacteriophage. This is in accordance with other studies reporting that plasma treatment could acidify distilled water (Choi et al., 2019; Xu et al., 2016). It is also reported by Shen et al. (2016) that ROS and low pH show a the synergistic bactericidal activity. According to them, lower pH helps to inactivate microbial cells by allowing reactive species in PAW to penetrate the cells and this highlights the synergistic effect reactive species and the acidity in PAW (Ma et al., 2015).
Conclusion
For a decontamination process to be considered effective, a reduction in bacterial counts of at least 3-logs is required, ideally a reduction of at least 5-logs should be observed. Fresh products can easily be contaminated with pathogens due to production conditions, fertilizer and irrigation water, and processing steps. In the current study, we evaluated that individual and combined effect of phage and PAW treatment to control Salmonella on fresh lettuce. According to our results, the strongest antimicrobial effect was obtained in the group treated with phage first following by PAW. At low inoculation level, phage + PAW reduced the bacterial count below the detection limit. It appears to be phage, and PAW combination can be used to eliminate pathogen bacteria at laboratory scale. Further optimization processes are required to enable the use of phage and PAW in a large scale of production.
Data Availability
No datasets were generated or analyzed during the current study.
References
Agun, L., Ahmad, N., Redzuan, N., Idirs, N. A. S., Taib, S. M., Zakaria, Z., & Ibrahim, R. K. R. (2021). Sterilization of oyster mushroom crop residue substrate by using cold plasma technology. Materials Today: Proceedings, 39, 903–906.
Bartz, F. E., Lickness, J. S., Heredia, N., Fabiszewski de Aceituno, A., Newman, K. L., Hodge, D. W., et al. (2017). Contamination of fresh produce by microbial indicators on farms and in packing facilities: Elucidation of environmental routes. Applied and Environmental Microbiology, 83(11), e02984-e3016.
Bennett, S. D., Littrell, K. W., Hill, T. A., Mahovic, M., & Behravesh, C. B. (2015). Multistate foodborne disease outbreaks associated with raw tomatoes, United States, 1990–2010: A recurring public health problem. Epidemiology & Infection, 143(7), 1352–1359.
Bennett, S. D., Sodha, S. V., Ayers, T. L., Lynch, M. F., Gould, L. H., & Tauxe, R. V. (2018). Produce-associated foodborne disease outbreaks, USA, 1998–2013. Epidemiology & Infection, 146(11), 1397–1406.
BIOHAZ, E. P. on B. H. (2016). Evaluation of the safety and efficacy of Listex™ P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA Journal, 14(8), e04565.
Chanishvili, N. (2012). Phage therapy—history from Twort and d’Herelle through Soviet experience to current approaches. Advances in Virus Research, 83, 3–40.
Charoux, C. M. G., Patange, A. D., Hinds, L. M., Simpson, J. C., O’Donnell, C. P., & Tiwari, B. K. (2020). Antimicrobial effects of airborne acoustic ultrasound and plasma activated water from cold and thermal plasma systems on biofilms. Scientific Reports, 10(1), 17297.
Choi, E. J., Park, H. W., Kim, S. B., Ryu, S., Lim, J., Hong, E. J., et al. (2019). Sequential application of plasma-activated water and mild heating improves microbiological quality of ready-to-use shredded salted kimchi cabbage (Brassica pekinensis L.). Food Control, 98, 501–509.
Choińska-Pulit, A., Mituła, P., Śliwka, P., Łaba, W., & Skaradzińska, A. (2015). Bacteriophage encapsulation: Trends and potential applications. Trends in Food Science & Technology, 45(2), 212–221. https://doi.org/10.1016/j.tifs.2015.07.001
Cong, K.-P., Li, T.-T., Wu, C.-E., Zeng, K.-F., Zhang, J.-H., Fan, G.-J., et al. (2022). Effects of plasma-activated water on overall quality of fresh goji berries during storage. Scientia Horticulturae, 293, 110650.
Dasan, B. G., & Boyaci, I. H. (2018). Effect of cold atmospheric plasma on inactivation of Escherichia coli and physicochemical properties of apple, orange, tomato juices, and sour cherry nectar. Food and Bioprocess Technology, 11(2), 334–343.
Dasan, B. G., Boyaci, I. H., & Mutlu, M. (2016). Inactivation of aflatoxigenic fungi (Aspergillus spp.) on granular food model, maize, in an atmospheric pressure fluidized bed plasma system. Food Control, 70, 1–8.
Ekiz, E., Tayyarcan, E. K., Evran, E., Guven, K., Soykut, E. A., & Boyaci, I. H. (2023). Investigation of the effect of bacteriophage cocktail on microbial quality in the case of cold chain breakage: A case study on Escherichia coli contamination in milk. Food and Humanity, 1, 1073–1081.
Evran, S., Tayyarcan, E. K., Acar-Soykut, E., & Boyaci, I. H. (2022). Applications of bacteriophage cocktails to reduce Salmonella contamination in poultry farms. Food and Environmental Virology, 14(1), 1–9.
Gavahian, M., Sheu, F., Tsai, M., & Chu, Y. (2020). The effects of dielectric barrier discharge plasma gas and plasma-activated water on texture, color, and bacterial characteristics of shiitake mushroom. Journal of Food Processing and Preservation, 44(1), e14316.
Gil, M. I., Selma, M. V., López-Gálvez, F., & Allende, A. (2009). Fresh-cut product sanitation and wash water disinfection: Problems and solutions. International Journal of Food Microbiology, 134(1–2), 37–45.
Gordillo Altamirano, F. L., & Barr, J. J. (2019). Phage therapy in the postantibiotic era. Clinical Microbiology Reviews, 32(2), e00066–e118.
Gragg, S. E., & Brashears, M. M. (2010). Reduction of Escherichia coli O157: H7 in fresh spinach, using lactic acid bacteria and chlorine as a multihurdle intervention. Journal of Food Protection, 73(2), 358–361.
Guo, J., Huang, K., Wang, X., Lyu, C., Yang, N., Li, Y., & Wang, J. (2017). Inactivation of yeast on grapes by plasma-activated water and its effects on quality attributes. Journal of Food Protection, 80(2), 225–230.
Guo, Y., Li, J., Islam, M. S., Yan, T., Zhou, Y., Liang, L., et al. (2021). Application of a novel phage vB_SalS-LPSTLL for the biological control of Salmonella in foods. Food Research International, 147, 110492.
Han, J.-Y., Song, W.-J., Kang, J. H., Min, S. C., Eom, S., Hong, E. J., et al. (2020). Effect of cold atmospheric pressure plasma-activated water on the microbial safety of Korean rice cake. LWT, 120, 108918.
Han, Q.-Y., Wen, X., Gao, J.-Y., Zhong, C.-S., & Ni, Y.-Y. (2022). Application of plasma-activated water in the food industry: A review of recent research developments. Food Chemistry, 134797.
Hou, C.-Y., Lai, Y.-C., Hsiao, C.-P., Chen, S.-Y., Liu, C.-T., Wu, J.-S., & Lin, C.-M. (2021). Antibacterial activity and the physicochemical characteristics of plasma activated water on tomato surfaces. Lwt, 149, 111879.
Islam, M., Zhou, Y., Liang, L., Nime, I., Liu, K., Yan, T., et al. (2019). Application of a phage cocktail for control of Salmonella in foods and reducing biofilms. Viruses, 11(9), 841.
Kaushik, N. K., Ghimire, B., Li, Y., Adhikari, M., Veerana, M., Kaushik, N., et al. (2019). Biological and medical applications of plasma-activated media, water and solutions. Biological Chemistry, 400(1), 39–62.
Leverentz, B., Conway, W. S., Alavidze, Z., Janisiewicz, W. J., Fuchs, Y., Camp, M. J., et al. (2001). Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit: A model study. Journal of Food Protection, 64(8), 1116–1121.
Lim, J., Byeon, Y., Hong, E. J., Ryu, S., & Kim, S. B. (2021). Effect of post-discharge time of plasma-treated water (PTW) on microbial inactivation and quality of fresh-cut potatoes. Journal of Food Processing and Preservation, 45(5), e15387.
Liu, C., Chen, C., Jiang, A., Sun, X., Guan, Q., & Hu, W. (2020). Effects of plasma-activated water on microbial growth and storage quality of fresh-cut apple. Innovative Food Science & Emerging Technologies, 59, 102256.
Lin, C.-M., Chu, Y.-C., Hsiao, C.-P., Wu, J.-S., Hsieh, C.-W., & Hou, C.-Y. (2019). The optimization of plasma-activated water treatments to inactivate Salmonella enteritidis (ATCC 13076) on shell eggs. Foods, 8(10), 520.
Lin, C.-M., Hsiao, C.-P., Lin, H.-S., Liou, J. S., Hsieh, C.-W., Wu, J.-S., & Hou, C.-Y. (2020). The antibacterial efficacy and mechanism of plasma-activated water against salmonella enteritidis (ATCC 13076) on shell eggs. Foods, 9(10), 1491.
Lone, A., Anany, H., Hakeem, M., Aguis, L., Avdjian, A.-C., Bouget, M., et al. (2016). Development of prototypes of bioactive packaging materials based on immobilized bacteriophages for control of growth of bacterial pathogens in foods. International Journal of Food Microbiology, 217, 49–58.
Luna-Guevara, J. J., Arenas-Hernandez, M. M. P., Martínez de la Peña, C., Silva, J. L., & Luna-Guevara, M. L. (2019). The role of pathogenic E. coli in fresh vegetables: Behavior, contamination factors, and preventive measures. International Journal of Microbiology, 2019, 2894328.
Ma, M., Zhang, Y., Lv, Y., & Sun, F. (2020). The key reactive species in the bactericidal process of plasma activated water. Journal of Physics d: Applied Physics, 53(18), 185207.
Ma, R., Wang, G., Tian, Y., Wang, K., Zhang, J., & Fang, J. (2015). Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. Journal of Hazardous Materials, 300, 643–651.
Ma, R., Yu, S., Tian, Y., Wang, K., Sun, C., Li, X., et al. (2016). Effect of non-thermal plasma-activated water on fruit decay and quality in postharvest Chinese bayberries. Food and Bioprocess Technology, 9, 1825–1834.
Machala, Z., Tarabová, B., Sersenová, D., Janda, M., & Hensel, K. (2018). Chemical and antibacterial effects of plasma activated water: Correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. Journal of Physics d: Applied Physics, 52(3), 34002.
Marcó, M. B., Moineau, S., & Quiberoni, A. (2012). Bacteriophages and Dairy Fermentations. Bacteriophage, 2(3), 149–158. https://doi.org/10.4161/bact.21868
Misra, N. N., Patil, S., Moiseev, T., Bourke, P., Mosnier, J. P., Keener, K. M., & Cullen, P. J. (2014). In-package atmospheric pressure cold plasma treatment of strawberries. Journal of Food Engineering, 125, 131–138.
Modi, R., Hirvi, Y., Hill, A., & Griffiths, M. W. (2001). Effect of phage on survival of Salmonella enteritidis during manufacture and storage of cheddar cheese made from raw and pasteurized milk. Journal of Food Protection, 64(7), 927–933.
Muhammad, A. I., Chen, W., Liao, X., Xiang, Q., Liu, D., Ye, X., & Ding, T. (2019). Effects of plasma-activated water and blanching on microbial and physicochemical properties of tiger nuts. Food and Bioprocess Technology, 12, 1721–1732.
Oliveira, M., Vi, I., ColÓs, P., Anguera, M., Usall, J., & Abadias, M. (2014). Effectiveness of a bacteriophage in reducing Listeria monocytogenes on fresh-cut fruits and fruit juices. Food Microbiology, 38, 137–142.
Pan, Y., Cheng, J., & Sun, D. (2019). Cold plasma-mediated treatments for shelf life extension of fresh produce: A review of recent research developments. Comprehensive Reviews in Food Science and Food Safety, 18(5), 1312–1326.
Pankaj, S. K., & Keener, K. M. (2017). Cold plasma: Background, applications and current trends. Current Opinion in Food Science, 16, 49–52.
Patange, A., Lu, P., Boehm, D., Cullen, P. J., & Bourke, P. (2019). Efficacy of cold plasma functionalised water for improving microbiological safety of fresh produce and wash water recycling. Food Microbiology, 84, 103226.
Perinban, S., Orsat, V., Lyew, D., & Raghavan, V. (2022). Effect of plasma activated water on Escherichia coli disinfection and quality of kale and spinach. Food Chemistry, 397, 133793.
Phongtang, W., Choi, G.-P., Chukeatirote, E., & Ahn, J. (2019). Bacteriophage control of Salmonella Typhimurium in milk. Food Science and Biotechnology, 28, 297–301.
Risa Vaka, M., Sone, I., García Álvarez, R., Walsh, J. L., Prabhu, L., Sivertsvik, M., & Noriega Fernández, E. (2019). Towards the next-generation disinfectant: Composition, storability and preservation potential of plasma activated water on baby spinach leaves. Foods, 8(12), 692.
Shah, U., Wang, Q., Kathariou, S., & Salvi, D. (2023). Optimization of Plasma-activated water and validation of a potential surrogate for Salmonella for future egg washing processes. Journal of Food Protection, 86(1), 100029.
Shang, H., Huang, L., Stanley, R., Deaker, R., & Bowman, J. P. (2023). The efficacy of preharvest application of electrolyzed water and chemical sanitizers against foodborne pathogen surrogates on leafy green vegetables. Journal of Food Safety, 43(4), e13051.
Shen, J., Tian, Y., Li, Y., Ma, R., Zhang, Q., Zhang, J., & Fang, J. (2016). Bactericidal effects against S. aureus and physicochemical properties of plasma activated water stored at different temperatures. Scientific Reports, 6, 28505.
Sillankorva, S. M., Oliveira, H., & Azeredo, J. (2012). Bacteriophages and their role in food safety. International Journal of Microbiology, 2012, 863945.
Smet, C., Govaert, M., Kyrylenko, A., Easdani, M., Walsh, J. L., & Van Impe, J. F. (2019). Inactivation of single strains of Listeria monocytogenes and Salmonella typhimurium planktonic cells biofilms with plasma activated liquids. Frontiers in Microbiology, 10, 1539.
Snyder, A. B., Perry, J. J., & Yousef, A. E. (2016). Developing and optimizing bacteriophage treatment to control enterohemorrhagic Escherichia coli on fresh produce. International Journal of Food Microbiology, 236, 90–97.
Soykut, E. A., Tayyarcan, E. K., Evran, Ş., Boyacı, İ. H., Çakır, İ., Khaaladi, M., & Fattouch, S. (2019). Microencapsulation of phages to analyze their demeanor in physiological conditions. Folia Microbiologica (Praha), 64(6), 751–763.
Stachler, E., Kull, A., & Julian, T. R. (2021). Bacteriophage treatment before chemical disinfection can enhance removal of plastic-surface-associated Pseudomonas aeruginosa. Applied and Environmental Microbiology, 87(20), e00980-e1021.
Tayyarcan, E. K., Evran, S., Akin, P. A., Soykut, E. A., & Boyaci, I. H. (2022). The use of bacteriophage cocktails to reduce Salmonella Enteritidis in hummus. LWT, 154, 112848.
Thirumdas, R., Kothakota, A., Annapure, U., Siliveru, K., Blundell, R., Gatt, R., & Valdramidis, V. P. (2018). Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends in Food Science & Technology, 77, 21–31.
Van Haute, S., Sampers, I., Holvoet, K., & Uyttendaele, M. (2013). Physicochemical quality and chemical safety of chlorine as a reconditioning agent and wash water disinfectant for fresh-cut lettuce washing. Applied and Environmental Microbiology, 79(9), 2850–2861.
Vengarai Jagannathan, B., Kitchens, S., Priyesh Vijayakumar, P., Price, S., & Morgan, M. (2021). Efficacy of bacteriophage cocktail to control E. coli O157: H7 contamination on baby spinach leaves in the presence or absence of organic load. Microorganisms, 9(3), 544.
Verraes, C., Van Boxstael, S., Van Meervenne, E., Van Coillie, E., Butaye, P., Catry, B., et al. (2013). Antimicrobial resistance in the food chain: A review. International Journal of Environmental Research and Public Health, 10(7), 2643–2669.
Wang, J., Han, R., Liao, X., & Ding, T. (2021). Application of plasma-activated water (PAW) for mitigating methicillin-resistant Staphylococcus aureus (MRSA) on cooked chicken surface. Lwt, 137, 110465.
Williams, M. S., Ebel, E. D., Saini, G., & Nyirabahizi, E. (2020). Changes in Salmonella contamination in meat and poultry since the introduction of the pathogen reduction and hazard analysis and critical control point rule. Journal of Food Protection, 83(10), 1707–1717.
Xiang, Q., Kang, C., Niu, L., Zhao, D., Li, K., & Bai, Y. (2018). Antibacterial activity and a membrane damage mechanism of plasma-activated water against Pseudomonas deceptionensis CM2. Lwt, 96, 395–401.
Xu, Y., Tian, Y., Ma, R., Liu, Q., & Zhang, J. (2016). Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chemistry, 197, 436–444.
Yesil, M., Kasler, D. R., Huang, E., & Yousef, A. E. (2023). Lytic Escherichia phage OSYSP acts additively and synergistically with gaseous ozone against Escherichia coli O157: H7 on spinach leaves. Scientific Reports, 13(1), 10706.
Zhao, Y., Chen, R., Liu, D., Wang, W., Niu, J., Xia, Y., et al. (2019). Effect of nonthermal plasma-activated water on quality and antioxidant activity of fresh-cut kiwifruit. IEEE Transactions on Plasma Science, 47(11), 4811–4817.
Zhao, Y.-M., Oliveira, M., Burgess, C. M., Cropotova, J., Rustad, T., Sun, D.-W., & Tiwari, B. K. (2021). Combined effects of ultrasound, plasma-activated water, and peracetic acid on decontamination of mackerel fillets. Lwt, 150, 111957.
Zhao, Y.-M., Patange, A., Sun, D., & Tiwari, B. (2020). Plasma-activated water: Physicochemical properties, microbial inactivation mechanisms, factors influencing antimicrobial effectiveness, and applications in the food industry. Comprehensive Reviews in Food Science and Food Safety, 19(6), 3951–3979.
Zinno, P., Devirgiliis, C., Ercolini, D., Ongeng, D., & Mauriello, G. (2014). Bacteriophage P22 to challenge Salmonella in foods. International Journal of Food Microbiology, 191, 69–74.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
Eylul Evran: Investigation, Data curation, Writing original draft, Review and editing, Visualization. Beyhan Gunaydin Dasan: Conceptualization, Methodology, Investigation, Writing original draft, Visualization, Review and editing. Emine Kubra Tayyarcan: Investigation, Writing original draft, Review and editing, Visualization. Ismail Hakki Boyaci: Conceptualization, Methodology, Review and editing, Supervision.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Evran, E., Dasan, B.G., Tayyarcan, E.K. et al. Effect of Sequential Treatment of Plasma Activated Water and Bacteriophage on Decontamination of Salmonella Typhimurium in Lettuce. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03355-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03355-7