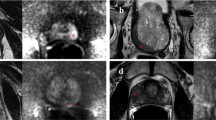
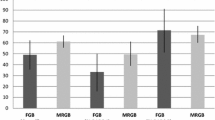
Abstract
Purpose of Review
The goal of this study is to review recent findings and evaluate the utility of MRI transrectal ultrasound fusion biopsy (FBx) techniques and discuss future directions.
Recent Findings
FBx detects significantly higher rates of clinically significant prostate cancer (csPCa) than ultrasound-guided systematic prostate biopsy (SBx), particularly in repeat biopsy settings. FBx has also been shown to detect significantly lower rates of clinically insignificant prostate cancer. In addition, a dedicated prostate MRI can assist in more accurately predicting the Gleason score and provide further information regarding the index cancer location, prostate volume, and clinical stage. The ability to accurately evaluate specific lesions is vital to both focal therapy and active surveillance, for treatment selection, planning, and adequate follow-up.
Summary
FBx has been demonstrated in multiple high-quality studies to have improved performance in diagnosis of csPCa compared to SBx. The combination of FBx with novel technologies including radiomics, prostate-specific membrane antigen positron emission tomography (PSMA PET), and high-resolution micro-ultrasound may have the potential to further enhance this performance.

Similar content being viewed by others
Data Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22:2141–9. https://doi.org/10.1200/JCO.2004.10.062.
• Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22. https://doi.org/10.1016/S0140-6736(16)32401-1This is a multicenter international paired validating study which evaluates biopsy-naïve men with mpMRI followed by TRUS-SBx and template mapping biopsy, determining that mpMRI is more sensitive and less specific than TRUS-SBx for detection of csPCa.
Noguchi M, Stamey TA, McNeal JE, Yemoto CM. Relationship between systematic biopsies and histological features of 222 radical prostatectomy specimens: lack of prediction of tumor significance for men with nonpalpable prostate cancer. J Urol. 2001;166:104–10. https://doi.org/10.1016/S0022-5347(05)66086-7.
Crawford ED, Wilson SS, Torkko KC, et al. Clinical staging of prostate cancer: a computer-simulated study of transperineal prostate biopsy. BJU Int. 2005;96:999–1004. https://doi.org/10.1111/j.1464-410X.2005.05801.x.
Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol. 2014;66:343–51. https://doi.org/10.1016/j.eururo.2013.10.048.
Hoeks CMA, Schouten MG, Bomers JGR, et al. Three-tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol. 2012;62:902–9. https://doi.org/10.1016/j.eururo.2012.01.047.
•• EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. 2015. https://uroweb.org/wp-content/uploads/EAU-EANM-ESTRO-ESUR-SIOG-Guidelines-on-Prostate-Cancer-2020v4.pdf Accessed 1 Oct 2020. EAU Guidelines on Prostate Cancer recommend performing mpMRI before PBx in biopsy naïve or prior negative patients.
•• Prostate cancer: diagnosis and management NICE guideline. 2019. https://www.nice.org.uk/guidance/ng131/resources/prostate-cancer-diagnosis-and-management-pdf-66141714312133 Accessed 1 Oct 2020. NICE Guidelines on prostate cancer recommend performing mpMRI as the first-line investigation for people with suspected localised csPCa.
•• NCCN Guidelines Version 2.2019 Prostate Cancer Early Detection. 2019. https://www2.tri-kobe.org/nccn/guideline/urological/english/prostate_detection.pdf Accessed 1 Oct 2020. NCCN Guidelines recommend the use of MRI and MRI-TBx can be considered in addition to TRUS-SBx in the PBx-naive men and MRI-TBx should be considered in prior negative PBx and persistent cancer suspicious cases.
•• Bjurlin MA, Carroll PR, Eggener S, et al. Update of the standard operating procedure on the use of multiparametric magnetic resonance imaging for the diagnosis, staging and management of prostate cancer. J Urol. 2020;203:706–12. https://doi.org/10.1097/ju.0000000000000617AUA Guidelines recommend pre PBx MRI in men at risk for harboring PCa both in initial PBx men and prior negative PBx men with an increasing PSA.
•• Drost F-JH, Osses DF, Nieboer D, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev. 2019;4(4):CD012663. https://doi.org/10.1002/14651858.CD012663.pub2The study is a meta-analysis based on 43 studies. This study demonstrated the high sensitivity for clinical significant cancer and low sensitivity for clinically insignificant cancer of MR image guided targeted biopsy.
• Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–77. https://doi.org/10.1056/NEJMoa1801993This multicenter randomized noninferiority trial compared a MRI-only pathway to TRUS-SBx, finding that CSPCa detection rates were higher in the MRI-targeted biopsy group, and that over one quarter of the MRI-only men avoided biopsy altogether, providing evidence for an MRI-only pathway as a clinical alternative.
• Rouvière O, Puech P, Renard-Penna R, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019;20:100–9. https://doi.org/10.1016/S1470-2045(18)30569-2This prospective multicenter paired diagnostic study found that both targeted and systematic biopsy missed csPCa.
• van der Leest M, Cornel E, Israël B, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol. 2019;75:570–8. https://doi.org/10.1016/j.eururo.2018.11.023This multicenter prospective study provides further high-level evidence regarding low rates of underdetection of csPCa with MRI-TBx, as well as rates of overdetection of ciPCa with TRUS-SBx.
• Wegelin O, van Melick HHE, Hooft L, et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique? Eur Urol. 2017;71:517–31. https://doi.org/10.1016/j.eururo.2016.07.041This systematic review and meta-analysis evaluates patients that underwent MRI targeted biopsy and systematic biopsy within the same population, allowing the authors to evaluate the performance of MRI targeted biopsy against systematic biopsy, as well as evaluating different biopsy techniques.
• Kasivisvanathan V, Stabile A, Neves JB, et al. Magnetic resonance imaging-targeted biopsy versus systematic biopsy in the detection of prostate cancer: a systematic review and meta-analysis. Eur Urol. 2019;76:284–303. https://doi.org/10.1016/j.eururo.2019.04.043This systematic review and meta-analysis provides second level data describing the performance of MRI targeted biopsy compared to systematic biopsy in paired cohorts in which both biopsies were performed in the same patient, and finding no differences in prior biopsy status.
• Wegelin O, Exterkate L, van der Leest M, et al. The FUTURE trial: a multicenter randomised controlled trial on target biopsy techniques based on magnetic resonance imaging in the diagnosis of prostate cancer in patients with prior negative biopsies. Eur Urol. 2019;75:582–90. https://doi.org/10.1016/j.eururo.2018.11.040The study is a multicenter randomised controlled trial including 665 men with prior negative systematic biopsy. This study revealed there were no significant differences in the csPCa detection rates between FBx, In-bore PBx, and Cog-PBx.
• Elkhoury FF, Felker ER, Kwan L, et al. Comparison of targeted vs systematic prostate biopsy in men who are biopsy naive: the prospective assessment of image registration in the diagnosis of prostate cancer (PAIREDCAP) study. JAMA Surg. 2019;154:811–8. https://doi.org/10.1001/jamasurg.2019.1734This paired cohort trial evaluated biopsy naïve patients, demonstrating that detection of csPCa was highest when combining targeted and systematic biopsy.
Simmons LAM, Kanthabalan A, Arya M, et al. Accuracy of transperineal targeted prostate biopsies, visual estimation and image fusion in men needing repeat biopsy in the PICTURE trial. J Urol. 2018;200:1227–34. https://doi.org/10.1016/j.juro.2018.07.001.
Goldberg H, Ahmad AE, Chandrasekar T, et al. Comparison of magnetic resonance imaging and transrectal ultrasound informed prostate biopsy for prostate cancer diagnosis in biopsy naïve men: a systematic review and meta-analysis. J Urol. 2020;203:1085–93. https://doi.org/10.1097/ju.0000000000000595.
Porreca A, Del Giudice F, Giampaoli M, et al. Adding systematic biopsy to magnetic resonance ultrasound fusion targeted biopsy of the prostate in men with previous negative biopsy or enrolled in active surveillance programs: a prospective single center, randomized study. Medicine (Baltimore). 2020;e22059:99. https://doi.org/10.1097/MD.0000000000022059.
•• Ahdoot M, Wilbur AR, Reese SE, et al. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med. 2020;382:917–28. https://doi.org/10.1056/NEJMoa1910038The study is a single center prospective study including 2103 men who underwent prebiopsy MRI, with MRI-visible lesions, who underwent combined biopsy. This study revealed the upgrading rate of FBx was less than that of TRUS-SBx.
Kenigsberg AP, Renson A, Rosenkrantz AB, et al. Optimizing the number of cores targeted during prostate magnetic resonance imaging fusion target biopsy. Eur Urol Oncol. 2018;1:418–25. https://doi.org/10.1016/j.euo.2018.09.006.
Lu AJ, Syed JS, Ghabili K, et al. Role of core number and location in targeted magnetic resonance imaging-ultrasound fusion prostate biopsy. Eur Urol. 2019;76:14–7. https://doi.org/10.1016/j.eururo.2019.04.008.
• Hansen NL, Barrett T, Lloyd T, et al. Optimising the number of cores for magnetic resonance imaging-guided targeted and systematic transperineal prostate biopsy. BJU Int. 2020;125:260–9. https://doi.org/10.1111/bju.14865This study evaluates the optimum number of targeted and systematic cores necessary to allow for accurate diagnosis of prostate cancer.
Ahmed HU. The index lesion and the origin of prostate cancer. N Engl J Med. 2009;361:1704. https://doi.org/10.1056/NEJMcibr0905562.
Priester A, Natarajan S, Khoshnoodi P. al. Magnetic resonance imaging underestimation of prostate cancer geometry: use of patient specific molds to correlate images with whole mount pathology. J Urol. 2017;197:320–6. https://doi.org/10.1016/j.juro.2016.07.084.
Le Nobin J, Orczyk C, Deng FM, et al. Prostate tumour volumes: evaluation of the agreement between magnetic resonance imaging and histology using novel co-registration software. BJU Int. 2014;114:E105–12. https://doi.org/10.1111/bju.12750.
Baco E, Ukimura O, Rud E, et al. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index tumor: correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol. 2015;67:787–94. https://doi.org/10.1016/j.eururo.2014.08.077.
• Simopoulos DN, Sisk AE, Priester A, et al. Cancer core length from targeted biopsy: an index of prostate cancer volume and pathological stage. BJU Int. 2019;124:275–81. https://doi.org/10.1111/bju.14691The study is a single center retrospective study including 205 men who underwent FBx and RP. This study revealed the MCCL from target PBx correlated with index tumor volume, particularly in GG >3 index tumors, and was associated with pathologic T3 staging.
Matsugasumi T, Baco E, Palmer S, et al. Prostate cancer volume estimation by combining magnetic resonance imaging and targeted biopsy proven cancer core length: correlation with cancer volume. J Urol. 2015;194:957–65. https://doi.org/10.1016/j.juro.2015.04.075.
Feng TS, Sharif-Afshar AR, Wu J, et al. Multiparametric MRI improves accuracy of clinical nomograms for predicting extracapsular extension of prostate cancer. Urology. 2015;86:332–7. https://doi.org/10.1016/j.urology.2015.06.003.
Raskolnikov D, George AK, Rais-Bahrami S, et al. The role of magnetic resonance image guided prostate biopsy in stratifying men for risk of extracapsular extension at radical prostatectomy. J Urol. 2015;194:105–11. https://doi.org/10.1016/j.juro.2015.01.072.
•• Barnett CL, Davenport MS, Montgomery JS, Wei JT, Montie JE, Denton BT. Cost-effectiveness of magnetic resonance imaging and targeted fusion biopsy for early detection of prostate cancer. BJU Int. 2018;122:50–8. https://doi.org/10.1111/bju.14151This study demonstrated the cost effectiveness of FBx in biopsy naïve men using a Markov model. They found a PI-RADS threshold of score 3 gained more QALYs than a score of 4, and a combined PBx in case of positive MRI gained more QALYs than FBx alone.
Grey ADR, Chana MS, Popert R, Wolfe K, Liyanage SH, Acher PL. Diagnostic accuracy of magnetic resonance imaging (MRI) prostate imaging reporting and data system (PI-RADS) scoring in a transperineal prostate biopsy setting. BJU Int. 2015;115:728–35. https://doi.org/10.1111/bju.12862.
Gaziev G, Wadhwa K, Barrett T, et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusion-guided transperineal prostate biopsies as a validation tool. BJU Int. 2016;117:80–6. https://doi.org/10.1111/bju.12892.
Calio B, Sidana A, Sugano D, et al. Changes in prostate cancer detection rate of MRI-TRUS fusion vs systematic biopsy over time: evidence of a learning curve. Prostate Cancer Prostatic Dis. 2017;20:436–41. https://doi.org/10.1038/pcan.2017.34.
•• Halstuch D, Baniel J, Lifshitz D, Sela S, Ber Y, Margel D. Characterizing the learning curve of MRI-US fusion prostate biopsies. Prostate Cancer Prostatic Dis. 2019;22:546–51. https://doi.org/10.1038/s41391-019-0137-2This study is a single center prospective study including 523 transrectal and 256 transperineal FBx. The study describes the learning curve with regard to FBx procedure time.
• Tafuri A, Ashrafi AN, Palmer S, et al. One-stop MRI and MRI/transrectal ultrasound fusion-guided biopsy: an expedited pathway for prostate cancer diagnosis. World J Urol. 2020;38:949–56. https://doi.org/10.1007/s00345-019-02835-2This study evaluated the performance (i.e. cancer detection rate, complications, and negative predictive value) of mpMRI between one-stop (pre-biopsy mpMRI and PBx performed the same day) and standard two-visit pathways. The performance was not significantly different between one-stop and standard two-visit pathways.
Sathianathen NJ, Omer A, Harriss E, et al. Negative predictive value of multiparametric magnetic resonance imaging in the detection of clinically significant prostate cancer in the prostate imaging reporting and data system era: a systematic review and meta-analysis. Eur Urol. 2020;78:402–14. https://doi.org/10.1016/j.eururo.2020.03.048.
Washino S, Okochi T, Saito K, Konishi T, Hirai M, Kobayashi Y, et al. Combination of prostate imaging reporting and data system (PI-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int. 2017;119:225–33. https://doi.org/10.1111/bju.13465.
Hansen NL, Barrett T, Koo B, et al. The influence of prostate-specific antigen density on positive and negative predictive values of multiparametric magnetic resonance imaging to detect Gleason score 7–10 prostate cancer in a repeat biopsy setting. BJU Int. 2017;119:724–30. https://doi.org/10.1111/bju.13619.
• Hansen NL, Barrett T, Kesch C, et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy-naïve men with suspicion of prostate cancer. BJU Int. 2018;122:40–9. https://doi.org/10.1111/bju.14049This multicenter study evaluates the performance of MRI-guided transperineal biopsy, determining that this technique was able to obtain high detection rates of csPCa.
Oishi M, Shin T, Ohe C, et al. Which patients with negative magnetic resonance imaging can safely avoid biopsy for prostate cancer? J Urol. 2019;201:268–77. https://doi.org/10.1016/j.juro.2018.08.046.
•• Hale GR, Czarniecki M, Cheng A, et al. Comparison of elastic and rigid registration during magnetic resonance imaging/ultrasound fusion-guided prostate biopsy: a multi-operator phantom study. J Urol. 2018;200:1114–21. https://doi.org/10.1016/j.juro.2018.06.028This study is a direct comparison study of elastic and rigid registration error (RE) using 224 phantoms and a fusion biopsy system. The study revealed that there was no significant difference between elastic and rigid RE, and RE was lower with experienced operators. This result is valuable as it directly compares both methods in a controlled setting.
•• Venderink W, de Rooij M, Sedelaar JPM, Huisman HJ, Fütterer JJ. Elastic versus rigid image registration in magnetic resonance imaging–transrectal ultrasound fusion prostate biopsy: a systematic review and meta-analysis. Eur Urol Focus. 2018;4:219–27. https://doi.org/10.1016/j.euf.2016.07.003This study demonstrated the detection rate of csPCa was not significantly different between elastic and rigid registration methods.
Xiang J, Yan H, Li J, Wang X, Chen H, Zheng X. Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: a systematic review and meta-analysis. World J Surg Oncol. 2019;17:1–11. https://doi.org/10.1186/s12957-019-1573-0.
Loy LM, Lim GH, Leow JJ, Lee CH, Tan TW, Tan CH. A systematic review and meta-analysis of magnetic resonance imaging and ultrasound guided fusion biopsy of prostate for cancer detection—comparing transrectal with transperineal approaches. Urol Oncol Semin Orig Investig. 2020;38(8):650–60. https://doi.org/10.1016/j.urolonc.2020.04.005.
Pepe P, Garufi A, Priolo GD, Pennisi M. Multiparametric MRI/TRUS fusion prostate biopsy: advantages of a transperineal approach. Anticancer Res. 2017;37:3291–4. https://doi.org/10.21873/anticanres.11695.
Ukimura O, Abreu AL. Stereotactic, image-guided robotic biopsy. In: Polascik TJ, editor. Imaging and focal therapy of early prostate cancer. Imaging Focal Ther Early Prostate Cancer. New York: Springer; 2013. p. 119–32.
Ho HSS, Mohan P, Lim ED, et al. Robotic ultrasound-guided prostate intervention device: system description and results from phantom studies. Int J Med Robot Comput Assist Surg. 2009;5:51–8. https://doi.org/10.1002/rcs.232.
Ho H, Yuen JSP, Mohan P, Lim EW, Cheng CWS. Robotic transperineal prostate biopsy: pilot clinical study. Urology. 2011;78:1203–8. https://doi.org/10.1016/j.urology.2011.07.1389.
• Lee AY, Yang XY, Lee HJ, et al. Multiparametric MRI-ultrasound software fusion prostate biopsy - initial results using a stereotactic robotic assisted transperineal prostate biopsy platform comparing systematic versus targeted biopsy. BJU Int. 2020. https://doi.org/10.1111/bju.15118 Online ahead of print. This study is a single center retrospective study including 433 men underwent stereotactic robotic assisted transperineal biopsy. Although the study is retrospective, it provides important initial results of robotic biopsy.
• Yang XY, Lee AY, Law YM, et al. Stereotactic robot-assisted transperineal prostate biopsy under local anaesthesia and sedation: moving robotic biopsy from operating theatre to clinic. J Robot Surg. 2020;14:767–72. https://doi.org/10.1007/s11701-020-01052-zThis study is a single center prospective pilot study including 30 men who underwent stereotactic robotic assisted transperineal biopsy under local anesthesia and sedation for evaluating safety and feasibility. The study demonstrated robotic transperineal FBx can be safely and precisely performed.
Poquet C, Mozer P, Vitrani MA, Morel G. An endorectal ultrasound probe comanipulator with hybrid actuation combining brakes and motors. IEEE/ASME. Trans Mechatronics. 2015;20:186–96. https://doi.org/10.1109/TMECH.2014.2314859.
• Vitrani MA, Baumann M, Reversat D, Morel G, Moreau-Gaudry A, Mozer P. Prostate biopsies assisted by comanipulated probe-holder: first in man. Int J Comput Assist Radiol Surg. 2016;11:1153–61. https://doi.org/10.1007/s11548-016-1399-yThis study evaluates the in-vivo performance of a comanipulated probe holder, allowing for validation in the clinical setting.
Muntener M, Patriciu A, Petrisor D, et al. Transperineal prostate intervention: robot for fully automated MR imaging - system description and proof of principle in a canine model. Radiology. 2008;247:543–9. https://doi.org/10.1148/radiol.2472070737.
Goldenberg AA, Trachtenberg J, Yi Y, et al. Robot-assisted MRI-guided prostatic interventions. Robotica. 2010;28:215–34. https://doi.org/10.1017/S026357470999066X.
Van Den Bosch MR, Moman MR, Van Vulpen M, et al. MRI-guided robotic system for transperineal prostate interventions: proof of principle. Phys Med Biol. 2010;55:N133-40. https://doi.org/10.1088/0031-9155/55/5/N02.
Schouten MG, Bomers JGR, Yakar D, et al. Evaluation of a robotic technique for transrectal MRI-guided prostate biopsies. Eur Radiol. 2012;22:476–83. https://doi.org/10.1007/s00330-011-2259-3.
Krieger A, Song SE, Bongjoon Cho N, al. Development and evaluation of an actuated MRI-compatible robotic system for MRI-guided prostate intervention. IEEE/ASME Trans Mechatronics. 2013;18:273–284. DOI: https://doi.org/10.1109/TMECH.2011.2163523
Sridhar AN, Hughes-Hallett A, Mayer EK, et al. Image-guided robotic interventions for prostate cancer. Nat Rev Urol. 2013;10:452–62. https://doi.org/10.1038/nrurol.2013.129.
Stoianovici D, Kim C, Srimathveeravalli G, et al. MRI-safe robot for endorectal prostate biopsy. IEEE/ASME Trans Mechatronics. 2014;19:1289–1299. DOI: https://doi.org/10.1109/TMECH.2013.2279775
Su H, Shang W, Cole G, et al. Piezoelectrically actuated robotic system for MRI-guided prostate percutaneous therapy. IEEE/ASME Trans Mechatronics. 2015;20:1920–32. https://doi.org/10.1109/TMECH.2014.2359413.
Moreira P, van de Steeg G, Krabben T, et al. The MIRIAM robot: a novel robotic system for MR-guided needle insertion in the prostate. J Med Robot Res. 2017;02:1750006. https://doi.org/10.1142/S2424905X17500064.
• Venderink W, Bomers JG, Overduin CG, et al. Multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer: what urologists need to know. Part 3: targeted biopsy. Eur Urol. 2020;77:481–90. https://doi.org/10.1016/j.eururo.2019.10.009This study provides practical information on how to best optimize mpMRI image acquisition.
Zamecnik P, Schouten MG, Krafft AJ, et al. Automated real-time needle-guide tracking for fast 3-T MR-guided transrectal prostate biopsy: a feasibility study. Radiology. 2014;273:879–86. https://doi.org/10.1148/radiol.14132067.
Bomers JGR, Bosboom DGH, Tigelaar GH, Sabisch J, Fütterer JJ, Yakar D. Feasibility of a 2nd generation MR-compatible manipulator for transrectal prostate biopsy guidance. Eur Radiol. 2017;27:1776–82. https://doi.org/10.1007/s00330-016-4504-2.
Linder N, Schaudinn A, Petersen TO, et al. In-bore biopsies of the prostate assisted by a remote-controlled manipulator at 1.5 T. Magn Reson Mater Physics. Biol Med. 2019;32:599–605. https://doi.org/10.1007/s10334-019-00751-5.
Sugano D, Sanford D, Abreu A, Duddalwar V, Gill I, Cacciamani GE. Impact of radiomics on prostate cancer detection. Curr Opin Urol. 2020. https://doi.org/10.1097/MOU.0000000000000822 Online ahead of print.
Wright GL. Haley C, Beckett M Lou, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol Semin Orig Investig. 1995;1:18–28. https://doi.org/10.1016/1078-1439(95)00002-Y.
Perner S, Hofer MD, Kim R. al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38:696–701. https://doi.org/10.1016/j.humpath.2006.11.012.
•• Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised. multicentre study. Lancet. 2020;395:1208–16. https://doi.org/10.1016/S0140-6736(20)30314-7This prospective, randomized multicenter study demonstrated that PSMA PET/CT had greater accuracy, sensitivity, and specificity compared to conventional imaging for pelvic nodal or metastatic disease in prostate cancer. This study provides robust evidence supporting the role of PSMA PET/CT in evaluation of metastatic prostate cancer.
• Satapathy S, Singh H, Kumar R, Mittal BR. Diagnostic accuracy of 68 Ga-PSMA PET/CT for initial detection in patients with suspected prostate cancer: a systematic review and meta-analysis. Am J Roentgenol. 2020; DOI: 10.2214/AJR.20.23912. Online ahead of print. This meta-analysis evaluates the performance of PSMA PET/CT in the initial detection of prostate cancer.
Laurence Klotz CM. Can high resolution micro-ultrasound replace MRI in the diagnosis of prostate cancer? Eur Urol Focus. 2020;6:419–23. https://doi.org/10.1016/j.euf.2019.11.006.
Ghai S, Eure G, Fradet V, et al. Assessing cancer risk on novel 29 MHz micro-ultrasound images of the prostate: creation of the micro-ultrasound protocol for prostate risk identification. J Urol. 2016;196:562–9. https://doi.org/10.1016/j.juro.2015.12.093.
• Zhang M, Wang R, Wu Y, et al. Micro-ultrasound imaging for accuracy of diagnosis in clinically significant prostate cancer: a meta-analysis. Front Oncol. 2019;9:1–8. https://doi.org/10.3389/fonc.2019.01368This meta-analysis evaluates pooled data on the novel imaging modality high resolution micro-ultrasound. This second-level data allows clinicians to decide how to incorporate this into diagnostic pathways.
• Wiemer L, Hollenbach M, Heckmann R, et al. Evolution of targeted prostate biopsy by adding microultrasound to the magnetic resonance imaging pathway. Eur Urol Focus. 2020;S2405-4569(20):30188-7. https://doi.org/10.1016/j.euf.2020.06.022 Epub ahead of print. This study demonstrated that high resolution micro-ultrasound may allow for increased diagnostic accuracy when combined with mpMRI, possibly allowing clinicians to avoid systematic biopsy.
Code Availability
Not applicable.
Funding
This study is PARTIALLY supported by NIH (National Institutes of Health)/NCI (National Cancer Institute) Grant R01 CA205058-01 (ISG, ALA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Masatomo Kaneko, Dordaneh Sugano, Amir H Lebastchi, Jamal Nabhani, Christopher Haiman, and Giovanni E. Cacciamani each declare no potential conflicts of interest. Vinay A. Duddalwar is a Consultant for Radmetrix and Advisory Board Member for DeepTek Inc. Inderbir S. Gill is an Unpaid Advisor for Steba Biotech. Andre Luis Abreu is Consulting Physician for Koelis and was Training Proctor for Steba Biotech.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Surgery
Rights and permissions
About this article
Cite this article
Kaneko, M., Sugano, D., Lebastchi, A.H. et al. Techniques and Outcomes of MRI-TRUS Fusion Prostate Biopsy. Curr Urol Rep 22, 27 (2021). https://doi.org/10.1007/s11934-021-01037-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s11934-021-01037-x