Abstract
Summary
DeepDXA is a deep learning model designed to infer bone mineral density data from plain pelvis X-ray, and it can achieve good predicted value for clinical use.
Purpose
Osteoporosis is defined as a systemic disease of the bone characterized by a decrease in bone strength and deterioration of bone structure at the microscopic level, leading to bone fragility and increased risk of fracture. Bone mineral density (BMD) is the preferred method for the diagnosis of osteoporosis, and dual-energy x-ray absorptiometry (DXA) is the gold standard for diagnosing osteoporosis. Conventional radiography is more suited for the screening of osteoporosis rather than diagnosis, and osteoporosis can be detected on radiographs by experienced physicians only. This study explored the possibility of predicting BMD relative to DXA using patient radiographs.
Methods
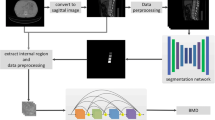
A deep learning algorithm of convolutional neural network (CNN) was used for the purpose. The method includes image segmentation, CNN learning, and a convolution-based regression model (DeepDXA) that links the isolated images of the femur bone to predict BMD value. Data were obtained in a single medical center from 2006 to 2018, with a total amount of 3472 pairs of pelvis X-ray and DXA examination within 1 year.
Results
The proposed workflow successfully predicted BMD values of the femur bone with the correlation coefficient (R) of 0.85 (P < 0.001) and the accuracy of 0.88 for prediction osteoporosis, a finding that could be reliably ready for further clinical use.
Conclusion
When suspicious osteoporosis is seen on plain films using the deep learning method we developed, further referral to DXA for the definite diagnosis of osteoporosis is indicated.



Similar content being viewed by others
References
Consensus A (1993) Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94(6):646–650
Solomon C, Black D, Rosen C (2016) Postmenopausal osteoporosis. N Engl J Med 374(3):254–262
Shao C-J, Hsieh Y-H, Tsai C-H, Lai K-A (2009) A nationwide seven-year trend of hip fractures in the elderly population of Taiwan. Bone 44(1):125–129
Choksi P, Jepsen KJ, Clines GA (2018) The challenges of diagnosing osteoporosis and the limitations of currently available tools. Clinical Diabetes and Endocrinology 4(1):12. https://doi.org/10.1186/s40842-018-0062-7
Keyak JH, Skinner HB, Fleming JA (2001) Effect of force direction on femoral fracture load for two types of loading conditions. J Orthop Res 19(4):539–544
Prevention O (2000) Diagnosis, and therapy. NIH Consens Statement 17(1):1–36
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos Int 4(6):368–381
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. The Lancet 359(9321):1929–1936
Kanis JA, Melton LJ III, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9(8):1137–1141
Baim S, Binkley N, Bilezikian JP, Kendler DL, Hans DB, Lewiecki EM, Silverman S (2008) Official positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom 11(1):75–91
Schousboe JT, Shepherd JA, Bilezikian JP, Baim S (2013) Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom 16 (4):455–466. doi:https://doi.org/10.1016/j.jocd.2013.08.004
Anil G, Guglielmi G, Peh WC (2010) Radiology of osteoporosis. Radiol Clin North Am 48(3):497–518. https://doi.org/10.1016/j.rcl.2010.02.016
Genant HK, Wu CY, Van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148
Nguyen BNT, Hoshino H, Togawa D, Matsuyama Y (2018) Cortical thickness index of the proximal femur: a radiographic parameter for preliminary assessment of bone mineral density and osteoporosis status in the age 50 years and over population. Clin Orthop Surg 10(2):149–156
He Q, Sun H, Shu L, Zhu Y, Xie X, Zhan Y, Luo C (2018) Radiographic predictors for bone mineral loss: cortical thickness and index of the distal femur. Bone & joint research 7(7):468–475
Clavert P, Javier R-M, Charrissoux J, Obert L, Pidhorz L, Sirveaux F, Mansat P, Fabre T (2016) How to determine the bone mineral density of the distal humerus with radiographic tools? Surg Radiol Anat 38(4):389–393
Samelson EJ, Broe KE, Xu H, Yang L, Boyd S, Biver E, Szulc P, Adachi J, Amin S, Atkinson E, Berger C, Burt L, Chapurlat R, Chevalley T, Ferrari S, Goltzman D, Hanley DA, Hannan MT, Khosla S, Liu C-T, Lorentzon M, Mellstrom D, Merle B, Nethander M, Rizzoli R, Sornay-Rendu E, Van Rietbergen B, Sundh D, Wong AKO, Ohlsson C, Demissie S, Kiel DP, Bouxsein ML (2019) Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the Bone Microarchitecture International Consortium (BoMIC): a prospective study. Lancet Diabetes Endocrinol 7(1):34–43. https://doi.org/10.1016/S2213-8587(18)30308-5
Vasikaran S, Eastell R, Bruyère O, Foldes A, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S, Trenti T (2011) Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 22(2):391–420
Johnell O, Odén A, De Laet C, Garnero P, Delmas P, Kanis J (2002) Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int 13(7):523
Garnero P, Sornay-Rendu E, Duboeuf F, Delmas PD (1999) Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study. J Bone Miner Res 14(9):1614–1621
Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP (2014) Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 29(3):518–530
Winzenrieth R, Michelet F, Hans D (2013) Three-dimensional (3D) microarchitecture correlations with 2D projection image gray-level variations assessed by trabecular bone score using high-resolution computed tomographic acquisitions: effects of resolution and noise. J Clin Densitom 16(3):287–296
Harvey NC, Glüer CC, Binkley N, McCloskey EV, Brandi ML, Cooper C, Kendler D, Lamy O, Laslop A, Camargos BM, Reginster JY, Rizzoli R, Kanis JA (2015) Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 78:216–224. https://doi.org/10.1016/j.bone.2015.05.016
Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg M-A (2011) Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 14(3):302–312. https://doi.org/10.1016/j.jocd.2011.05.005
Martineau P, Silva BC, Leslie WD (2017) Utility of trabecular bone score in the evaluation of osteoporosis. Curr Opin Endocrinol Diabetes Obes 24(6):402–410. https://doi.org/10.1097/med.0000000000000365
Silva BC, Leslie WD (2017) Trabecular bone score: a new DXA–derived measurement for fracture risk assessment. Endocrinol Metab Clin 46(1):153–180
Derkatch S, Kirby C, Kimelman D, Jozani MJ, Davidson JM, Leslie WD (2019) Identification of vertebral fractures by convolutional neural networks to predict nonvertebral and hip fractures: a registry-based cohort study of dual X-ray absorptiometry. Radiology 293(2):405–411
Melamed A, Vittinghoff E, Sriram U, Schwartz AV, Kanaya AM (2010) BMD reference standards among South Asians in the United States. J Clin Densitom 13(4):379–384. https://doi.org/10.1016/j.jocd.2010.05.007
Cundy T, Cornish J, Evans MC, Gamble G, Stapleton J, Reid IR (1995) Sources of interracial variation in bone mineral density. J Bone Miner Res 10(3):368–373
Lindner C, Thiagarajah S, Wilkinson JM, Wallis GA, Cootes TF, Consortium a (2013) Fully automatic segmentation of the proximal femur using random forest regression voting. IEEE transactions on medical imaging 32 (8):1462-1472
He K, Zhang X, Ren S, Sun J Deep residual learning for image recognition. In: Proceedings of the IEEE conference on computer vision and pattern recognition, 2016. pp 770–778
Simonyan K, Zisserman A (2014) Very deep convolutional networks for large-scale image recognition. arXiv preprint arXiv:14091556
Zhang H, Xue J, Dana K Deep ten: Texture encoding network. In: Proceedings of the IEEE conference on computer vision and pattern recognition, 2017. pp 708–717
Yu M, Tham Y-C, Rim TH, Ting DSW, Wong TY, Cheng C-Y (2019) Reporting on deep learning algorithms in health care. The Lancet Digital Health 1(7):e328–e329. https://doi.org/10.1016/S2589-7500(19)30132-3
Tecle N, Teitel J, Morris MR, Sani N, Mitten D, Hammert WC (2020) Convolutional neural network for second metacarpal radiographic osteoporosis screening. The Journal of Hand Surgery. https://doi.org/10.1016/j.jhsa.2019.11.019
Yasaka K, Akai H, Kunimatsu A, Kiryu S, Abe O (2020) Prediction of bone mineral density from computed tomography: application of deep learning with a convolutional neural network. Eur Radiol. https://doi.org/10.1007/s00330-020-06677-0
Liu J, Wang J, Ruan W, Lin C, Chen D (2019) Diagnostic and gradation model of osteoporosis based on improved deep U-Net network. J Med Syst 44(1):15. https://doi.org/10.1007/s10916-019-1502-3
Gay J, Harlin H (2019) Texture-based classification for oral cancer detection: implementation and performance analysis of deep learning approaches. Networks (RotEqNet) 8:11
Hu J, Song W, Zhang W, Zhao Y, Yilmaz A (2019) Deep learning for use in lumber classification tasks. Wood Sci Technol 53(2):505–517
Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S (2008) A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 23(2):205–214. https://doi.org/10.1359/jbmr.071020
Herrera A, Lobo-Escolar A, Mateo J, Gil J, Ibarz E, Gracia L (2012) Male osteoporosis: a review. World J Orthop 3(12):223–234. https://doi.org/10.5312/wjo.v3.i12.223
Wang L, Ran L, Zha X, Zhao K, Yang Y, Shuang Q, Liu Y, Hind K, Cheng X, Blake GM (2020) Adjustment of DXA BMD measurements for anthropometric factors and its impact on the diagnosis of osteoporosis. Arch Osteoporos 15(1):155. https://doi.org/10.1007/s11657-020-00833-1
Lochmüller EM, Miller P, Bürklein D, Wehr U, Rambeck W, Eckstein F (2000) In situ femoral dual-energy X-ray absorptiometry related to ash weight, bone size and density, and its relationship with mechanical failure loads of the proximal femur. Osteoporos Int 11(4):361–367. https://doi.org/10.1007/s001980070126
Boskey AL, Imbert L (2017) Bone quality changes associated with aging and disease: a review. Ann N Y Acad Sci 1410(1):93–106. https://doi.org/10.1111/nyas.13572
Fox KM, Magaziner J, Hawkes WG, Yu-Yahiro J, Hebel JR, Zimmerman SI, Holder L, Michael R (2000) Loss of bone density and lean body mass after hip fracture. Osteoporos Int 11(1):31–35. https://doi.org/10.1007/s001980050003
Acknowledgements
The authors thank the statistical assistance and wish to acknowledge the support of the Maintenance Project of the Center for Artificial Intelligence in Medicine at Chang Gung Memorial Hospital (Grant CLRPG3H0012, CIRPG3H0012) for study design and monitor, data analysis, and interpretation and Chang Gung Medical Foundation Grant (CMRPG5H0051-3, CMRPG3K0231-2) for manpower and data analysis.
Author information
Authors and Affiliations
Contributions
C.-S.H., Y.-P.C., T.-Y.F., C.-F.K., and Y.-C.P. designed research; C.-S.H., Y.-P.C., T.-Y.F., and T.-T.Y. collected data; C.-S.H., Y.-P.C., T.-Y.F., C.-F.K., and Y.-C.P. analyzed data; C.-S.H., Y.-P.C., Y.-C.L, and Y.-C.P. wrote the paper.
Corresponding authors
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ho, CS., Chen, YP., Fan, TY. et al. Application of deep learning neural network in predicting bone mineral density from plain X-ray radiography. Arch Osteoporos 16, 153 (2021). https://doi.org/10.1007/s11657-021-00985-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-021-00985-8