Abstract
Objectives
To investigate whether a deep learning model can predict the bone mineral density (BMD) of lumbar vertebrae from unenhanced abdominal computed tomography (CT) images.
Methods
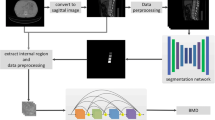
In this Institutional Review Board–approved retrospective study, patients who received both unenhanced CT examinations and dual-energy X-ray absorptiometry (DXA) of the lumbar vertebrae, in two institutions (1 and 2), were included. Supervised deep learning was employed to obtain a convolutional neural network (CNN) model using axial CT images, including the lumbar vertebrae as input data and BMD values obtained with DXA as reference data. For this purpose, 1665 CT images from 183 patients in institution 1, which were augmented to 99,900 (= 1665 × 60) images (noise adding, parallel shift and rotation were performed), were used. Internal (by using data of 45 other patients in institution 1) and external validations (by using data of 50 patients in institution 2) were performed to evaluate the performance of the trained CNN model. Correlations and diagnostic performances were evaluated with Pearson’s correlation coefficient (r) and area under the receiver operating characteristic curve (AUC), respectively.
Results
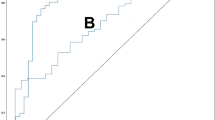
The estimated BMD values, according to the CNN model (BMDCNN), were significantly correlated with the BMD values obtained with DXA (r = 0.852 (p < 0.001) and 0.840 (p < 0.001) for the internal and external validation datasets, respectively). Using BMDCNN, osteoporosis was diagnosed with AUCs of 0.965 and 0.970 for the internal and external validation datasets, respectively.
Conclusions
Using deep learning, the BMD of lumbar vertebrae could be predicted from unenhanced abdominal CT images.
Key Points
• By applying a deep learning technique, the bone mineral density (BMD) of lumbar vertebrae can be estimated from unenhanced abdominal CT images.
• A strong correlation was observed between the estimated BMD and the BMD obtained with DXA.
• By using the estimated BMD, osteoporosis could be diagnosed with high performance.



Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the receiver operating characteristic curve
- BMD:
-
Bone mineral density
- BMDCNN :
-
Bone mineral density obtained with a convolutional neural network
- CNN:
-
Convolutional neural network
- CT:
-
Computed tomography
- DXA:
-
Dual-energy X-ray absorptiometry
- DICOM:
-
Digital imaging and communications in medicine
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
References
Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ 3rd (1993) Population-based study of survival after osteoporotic fractures. Am J Epidemiol 137:1001–1005
Hernlund E, Svedbom A, Ivergard M et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136
Kanis JA, Cooper C, Rizzoli R et al (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30:3–44
Compston J, Cooper A, Cooper C et al (2017) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 12:43
Orimo H, Nakamura T, Hosoi T et al (2012) Japanese 2011 guidelines for prevention and treatment of osteoporosis--executive summary. Arch Osteoporos 7:3–20
OECD Library (2017) Health at a glance 2017. Available via https://doi.org/10.1787/health_glance-2017-en. Accessed 20 Sept 2019
Bartalena T, Rinaldi MF, Modolon C et al (2010) Incidental vertebral compression fractures in imaging studies: lessons not learned by radiologists. World J Radiol 2:399–404
Krizhevsky A, Sutskever I, Hinton G (2012) ImageNet classification with deep convolutional neural networks. Advances in Neural Information Processing System 25 (NIPS 2012). Available via https://papers.nips.cc/paper/4824-imagenet-classification-with-deep-convolutional-neural-networks. Accessed 20 Dec 2017
Yasaka K, Abe O (2018) Deep learning and artificial intelligence in radiology: current applications and future directions. PLoS Med 15:e1002707
Chartrand G, Cheng PM, Vorontsov E et al (2017) Deep learning: a primer for radiologists. Radiographics 37:2113–2131
Yasaka K, Akai H, Kunimatsu A, Kiryu S, Abe O (2018) Deep learning with convolutional neural network in radiology. Jpn J Radiol 36:257–272
Zhu B, Liu JZ, Cauley SF, Rosen BR, Rosen MS (2018) Image reconstruction by domain-transform manifold learning. Nature 555:487–492
Yasaka K, Akai H, Abe O, Kiryu S (2018) Deep learning with convolutional neural network for differentiation of liver masses at dynamic contrast-enhanced CT: a preliminary study. Radiology 286:887–896
Kiryu S, Yasaka K, Akai H et al (2019) Deep learning to differentiate parkinsonian disorders separately using single midsagittal MR imaging: a proof of concept study. Eur Radiol 29:6891–6899
Urakawa T, Tanaka Y, Goto S, Matsuzawa H, Watanabe K, Endo N (2019) Detecting intertrochanteric hip fractures with orthopedist-level accuracy using a deep convolutional neural network. Skeletal Radiol 48:239–244
Derkatch S, Kirby C, Kimelman D, Jozani MJ, Davidson JM, Leslie WD (2019) Identification of vertebral fractures by convolutional neural networks to predict nonvertebral and hip fractures: a registry-based cohort study of dual X-ray absorptiometry. Radiology 293:405–411
Cheng CT, Ho TY, Lee TY et al (2019) Application of a deep learning algorithm for detection and visualization of hip fractures on plain pelvic radiographs. Eur Radiol 29:5469–5477
Yasaka K, Akai H, Kunimatsu A, Abe O, Kiryu S (2018) Liver fibrosis: deep convolutional neural network for staging by using gadoxetic acid-enhanced hepatobiliary phase MR images. Radiology 287:146–155
Yasaka K, Akai H, Kunimatsu A, Abe O, Kiryu S (2018) Deep learning for staging liver fibrosis on CT: a pilot study. Eur Radiol 28:4578–4585
Larson DB, Chen MC, Lungren MP, Halabi SS, Stence NV, Langlotz CP (2018) Performance of a deep-learning neural network model in assessing skeletal maturity on pediatric hand radiographs. Radiology. 287:313–322
Ioffe S, Szegedy C (2015) Batch normalization: accelerating deep network training by reducing internal covariate shift. Cornell University Library. Available via http://arxiv.org/abs/1502.03167. Accessed 30 April 2017
Kingma DP, Ba JL (2014) Adam: a method for stochastic optimization. Cornell University Library. Available via http://arxiv.org/abs/1412.6980. Accessed 30 April 2017
Schreiber JJ, Anderson PA, Hsu WK (2014) Use of computed tomography for assessing bone mineral density. Neurosurg Focus 37:E4
Hendrickson NR, Pickhardt PJ, Del Rio AM, Rosas HG, Anderson PA (2018) Bone mineral density T-scores derived from CT attenuation numbers (Hounsfield units): clinical utility and correlation with dual-energy X-ray absorptiometry. Iowa Orthop J 38:25–31
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Camacho PM, Petak SM, Binkley N et al (2016) American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis - 2016. Endocr Pract 22:1–42
Wood KB, Li W, Lebl DR, Ploumis A (2014) Management of thoracolumbar spine fractures. Spine J 14:145–164
Phillipov G, Seaborn CJ, Phillips PJ (2001) Reproducibility of DXA: potential impact on serial measurements and misclassification of osteoporosis. Osteoporos Int 12:49–54
Fuleihan GE, Testa MA, Angell JE, Porrino N, Leboff MS (1995) Reproducibility of DXA absorptiometry: a model for bone loss estimates. J Bone Miner Res 10:1004–1014
Schuit SC, van der Klift M, Weel AE et al (2004) Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone 34:195–202
Funding
This study has received funding by JSPS KAKENHI Grant Number JP18K15542.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Koichiro Yasaka.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Multicentre study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yasaka, K., Akai, H., Kunimatsu, A. et al. Prediction of bone mineral density from computed tomography: application of deep learning with a convolutional neural network. Eur Radiol 30, 3549–3557 (2020). https://doi.org/10.1007/s00330-020-06677-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06677-0