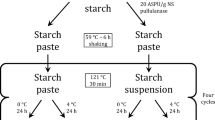
Abstract
The effect of starch-insoluble remnants (i.e., ghosts) on the behavior of the solid biopolymer electrolyte remains to be studied. This work focused on this issue by considering a case-study system formed by corn starch, glycerol, and lithium chloride. The ghost content was controlled by subjecting the gelatinized dispersion to ultrasonic cavitation at various times. Ghost content reduction led to a slight decrease in conductivity. When the ghost content was reduced to almost nil, the capacitance estimated from cyclic voltammetry tests showed a decrease of about 37%. In contrast, the stability of the electrolyte, estimated by repeated potential cycles, was positively affected. Formation of free radicals and starch chain retrogradation are postulated as the mechanisms involved in the conductivity and capacitance variations in corn starch, glycerol, and lithium chloride solid biopolymer electrolyte.








Similar content being viewed by others
References
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nanocomposite polymer electrolytes for lithium batteries. Nature 394:456–458
Meng C, Liu C, Chen L, Hu C, Fan S (2010) Highly flexible and all-solid-state paperlike polymer supercapacitors. Nano Lett 10(10):4025–4031
Wu JH, Lan Z, Lin JM, Huang ML, Hao SC, Sato T, Yin S (2007) A novel thermosetting gel electrolyte for stable quasi-solid-state dye-sensitized solar cells. Adv Mater 19:4006–4011
Khiar AA, Arof AK (2010) Conductivity studies of starch-based polymer electrolytes. Ionics 16:123–129
Khiar ASA, Puteh R, Arof AK (2006) Conductivity studies of a chitosan-based polymer electrolyte. Physica B 373:23–27
Yamazaki A, Takegawa R, Kaneko Y, Kadokawa JI, Yamagata M, Ishikawa M (2009) An acidic cellulose–chitin hybrid gel as novel electrolyte for an electric double layer capacitor. Electrochem Commun 11:68–70
Park SJ, Yoo K, Kim JY, Kim JY, Lee DK, Kim B, Ko MJ (2013) Water-based thixotropic polymer gel electrolyte for dye-sensitized solar cells. ACS Nano 7:4050–4056
Zobel HF (1988) Molecules to granules: a comprehensive starch review. Starch-Stärke 40:44–50
Marcondes RF, D'Agostini PS, Ferreira J, Girotto EM, Pawlicka A, Dragunski DC (2010) Amylopectin-rich starch plasticized with glycerol for polymer electrolyte application. Solid State Ionics 181:586–591
Sudhakar YN, Selvakumar M (2012) Lithium perchlorate doped plasticized chitosan and starch blend as biodegradable polymer electrolyte for supercapacitors. Electrochim Acta 78:398–405
Sudhakar YN, Selvakumar M (2013) Ionic conductivity studies and dielectric studies of poly(styrene sulphonic acid)/starch blend polymer electrolyte containing LiClO4. J Appl Electrochem 43:21–29
Kumar M, Tiwari T, Srivastava N (2012) Electrical transport behaviour of bio-polymer electrolyte system: potato starch + ammonium iodide. Carbohydr Polym 88:54–60
Lin Y, Li J, Liu K, Liu Y, Liu J, Wang X (2016) Unique starch polymer electrolyte for high capacity all-solid-state lithium sulfur battery. Green Chem 18:3796–5803
Biliaderis CG, Maurice TJ, Vose JR (1980) Starch gelatinization phenomena studied by differential scanning calorimetry. J Food Sci 45:1669–1674
Ratnayake WS, Jackson DS (2007) A new insight into the gelatinization process of native starches. Carbohydr Polym 67:511–529
Fannon JE, BeMiller JN (1992) Structure of corn starch paste and granule remnants revealed by low-temperature scanning electron microscopy after cryopreparation. Cereal Chem 69:456–460
Debet MR, Gidley MJ (2007) Why do gelatinized starch granules not dissolve completely? Roles for amylose, protein, and lipid in granule ‘ghost’ integrity. J Agric Food Chem 55:4752–4760
Zhang B, Dhital S, Flanagan BM, Gidley M (2014) Mechanism for starch granule ghost formation deduced from structural and enzyme digestion properties. J Agr Food Chem 62:760–771
Thiré RM, Simão RA, Andrade CT (2003) High resolution imaging of the microstructure of maize starch films. Carbohydr Polym 54:149–158
Garcia-Hernandez A, Vernon-Carter EJ, Alvarez-Ramirez J (2017) Impact of ghosts on the mechanical, optical, and barrier properties of corn starch films. Starch-Stärke. doi:10.1002/star.201600308
Ma X, Yu J, He K, Wang N (2007) Thermoplastic starch plasticized by glycerol as solid polymer electrolytes. Macromol Mat Eng 292:503–510
Ning W, Xingxiang Z, Haihui L, Jianping W (2009) N,N-dimethylacetamide/lithium chloride plasticized starch as solid biopolymer electrolytes. Carbohydr Polym 77:607–611
Wang N, Zhang X, Liu H, Han N (2010) Ionically conducting polymers based on ionic liquid-plasticized starch containing lithium chloride. Polym Polym Comp 18:53–58
Wexler A, Hazegawa S (1954) Relative humidity-temperature relationships of some saturated salt solutions in the temperature range 0 degree to 50 degrees C. J Res Natl Bur Stand 53:19–26
Atkin NJ, Abeysekera RM, Robards AW (1998) The events leading to the formation of ghost remnants from the starch granule surface and the contribution of the granule surface to the gelatinization endotherm. Carbohydr Polym 36:193–204
Jambrak AR, Herceg Z, Šubarić D, Babić J, Brnčić M, Brnčić SR, Gelo J (2010) Ultrasound effect on physical properties of corn starch. Carbohydr Polym 79:91–100
Czechowska-Biskup R, Rokita B, Lotfy S, Ulanski P, Rosiak JM (2005) Degradation of chitosan and starch by 360-kHz ultrasound. Carbohydr Polym 60:175–184
Zhu F (2015) Impact of ultrasound on structure, physicochemical properties, modifications, and applications of starch. Trends Food Sci Technol 43:1–17
Amini AM, Razavi SMA, Mortazavi SA (2015) Morphological, physicochemical, and viscoelastic properties of sonicated corn starch. Carbohydr Polym 122:282–292
Li G, Li Z, Zhang P, Zhang H, Wu Y (2008) Research on a gel polymer electrolyte for Li-ion batteries. Pure Appl Chem 80:2553–25563
Wang S, Li C, Copeland L, Niu Q, Wang S (2015) Starch retrogradation: a comprehensive review. Compr Rev Food Sci Food Saf 14:568–585
van Soest JJ, Tournois H, de Wit D, Vliegenthart JF (1995) Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr Res 279:201–214
Sevenou O, Hill SE, Farhat IA, Mitchell JR (2002) Organization of the external region of the starch granule as determined by infrared microscopy. Int J Biol Macromol 31:79–85
Jaipal Reddy M, Sreekanth T, Subba Rao UV (1999) Study of the plasticizer effect on a (PEO + NaYF4) polymer electrolyte and its use in an electrochemical cell. Solid State Ionics 126:55–63
Shukur MF, Kadir MFZ (2015) Hydrogen ion conducting starch-chitosan blend based electrolyte for application in electrochemical devices. Electrochim Acta 158:152–165
Bourtoom T (2008) Plasticizer effect on the properties of biodegradable blend film from rice starch-chitosan. J Sci Technol 30(Suppl. 1):149–165
Rice MJ, Roth WL (1972) Ionic transport in super ionic conductors: a theoretical model. J Solid State Chem 4(2):294–310
Beck M, Jekle M, Becker T (2011) Starch re-crystallization kinetics as a function of various cations. Starch-Stärke 63:792–800
Samsudin AS, Khairul WM, Isa MIN (2012) Characterization on the potential of carboxy methylcellulose for application as proton conducting biopolymer electrolytes. J Non-Cryst Solids 358:1104–1112
Malathi J, Kumaravadivel M, Brahmanandhan GM, Hema M, Baskaran R, Selvasekarapandian S (2010) Structural, thermal and electrical properties of PVA–LiCF3SO3 polymer electrolyte. J Non-Cryst Solids 356:2277–2281
Selvakumar M, Bhat DK (2008) LiClO4 doped cellulose acetate as biodegradable polymer electrolyte for supercapacitors. J Appl Polym Sci 110:594–602
Stephan AM, Thirunakaran RN, Renganathan G, Sundaram V, Pitchumani S, Muniyandi N, Ramamoorthy P (1999) A study on polymer blend electrolyte based on PVC/PMMA with lithium salt. J Power Sources 81:752–758
Lasia A (2014) Electrochemical impedance spectroscopy and its applications. Springer, New York
Sudhakar YN, Selvakumar M, Bhat DK (2015) Effect of acid dopants in biodegradable gel polymer electrolyte and the performance in an electrochemical double layer capacitor. Phys Scr 90:095702
Forssell P, Hamunen A, Autio K, Suortti P, Poutanen K (1995) Hypochlorite oxidation of barley and potato starch. Starch-Stärke 47:371–377
Acknowledgements
The authors thank the Consejo Nacional de Ciencia y Tecnología (CONACyT) for partially financing this work through project 236500.
Author contributions
C. Roldan-Cruz (Ph.D. student) designed and performed the EIS experiments. A. Garcia-Hernandez (Ph.D. student) obtained the optical and SEM micrographs and carried out the conductivity, opacity, and contact angle determinations. E. J. Vernon-Carter proposed the use of contact angle and EIS for monitoring film stability. J. Alvarez-Ramirez organized results and discussion. All authors contributed to the writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Roldan-Cruz, C., Garcia-Hernandez, A., Vernon-Carter, E.J. et al. Impact of insoluble starch remnants on the behavior of corn starch/glycerol/LiCl solid electrolyte. Ionics 23, 1721–1732 (2017). https://doi.org/10.1007/s11581-017-2014-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2014-0