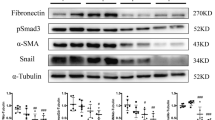
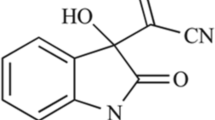
Abstract
Aristolochic acid (AA)-containing herbs have been prescribed for thousands of years as anti-inflammatory drugs, despite the active pharmaceutical ingredients remaining unclear. However, exposure to AAI and AAII has been proven to be a significant risk factor for severe nephropathy and carcinogenicity. AAIVa, an analogue abundant in AA-containing herbs, showed neither carcinogenicity nor nephrotoxicity in our study and other reports, implying that the pharmacological effects of AAIVa on inflammation are worth studying. Herein, we employed RAW 264.7 cells, the ear edema mouse model, and the lipopolysaccharide (LPS)-induced systematic inflammation model in TNF-IRES-Luc mice (tracking TNFα luciferase activities in real-time) to evaluate the anti-inframammary effect of AAIVa. Our results showed that AAIVa could decrease pro-inflammatory cytokines (TNFα and IL-6) production in LPS-stimulated RAW 264.7 cells, indicating its anti-inflammatory effects in vitro. Furthermore, the application of AAIVa (400 and 600 μg/ear) could significantly inhibit phorbol 12-myristate 13-acetate-induced ear edema, suggesting its topical anti-inflammatory activity in vivo. Moreover, LPS-stimulated TNF-IRES-Luc mice were used to investigate the onset and duration of AAIVa on systematic inflammation. A single dosage of AAIVa (100 mg/kg, i.g.) could suppress LPS-triggered inflammation, by decreasing luciferase activities of TNFα at 3 h in TNF-IRES-Luc mice. In addition, the online pharmacological databases predicted that AAIVa might target the regulation of T cell activation-related protein (ADA, ADORA2A, ERBB2) to exhibit anti-inflammatory effect. In conclusion, we demonstrated that AAIVa had anti-inflammatory effect for the first time; our findings are constructive for further studies on pharmacological mechanism of AAIVa.





Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Kumar V, Poonam PAK, Parmar VS (2003) Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities. Nat Prod Rep 20:565–583. https://doi.org/10.1039/b303648k
Heinrich M, Chan J, Wanke S, Neinhuis C, Simmonds MS (2009) Local uses of Aristolochia species and content of nephrotoxic aristolochic acid 1 and 2–a global assessment based on bibliographic sources. J Ethnopharmacol 125:108–144. https://doi.org/10.1016/j.jep.2009.05.028
Michl J, Ingrouille MJ, Simmonds MS, Heinrich M (2014) Naturally occurring aristolochic acid analogues and their toxicities. Nat Prod Rep 31:676–693. https://doi.org/10.1039/c3np70114j
Dey A, De JN (2011) Aristolochia indica L.: a review. Asian J Plant Sci 10:108–116. https://doi.org/10.3923/ajps.2011.108.116
Michl J, Jennings HM, Kite GC, Ingrouille MJ, Simmonds MS, Heinrich M (2013) Is aristolochic acid nephropathy a widespread problem in developing countries? A case study of Aristolochia indica L. in Bangladesh using an ethnobotanical-phytochemical approach. J Ethnopharmacol 149:235–244. https://doi.org/10.1016/j.jep.2013.06.028
Scarborough J (2011) Ancient medicinal use of Aristolochia: birthwort’s tradition and toxicity. Pharm Hist 53:3–21
Wu TS, Ou LF, Teng CM (1994) Aristolochic acids, aristolactam alkaloids and amides from Aristolochia kankauensis. Phytochemistry 36:1063–1068. https://doi.org/10.1016/s0031-9422(00)90492-8
Lerma-Herrera MA, Beiza-Granados L, Ochoa-Zarzosa A, Lopez-Meza JE, Navarro-Santos P, Herrera-Bucio R, Avina-Verduzco J, Garcia-Gutierrez HA (2022) Biological activities of organic extracts of the genus aristolochia: a review from 2005 to 2021. Molecules. https://doi.org/10.3390/molecules27123937
Moese JR (1963) Alteration of Infection Resistance by Aristolochic Acid. Langenbecks Arch Klin Chir Ver Dtsch Z Chir 304:657–660
Kupchan SM, Doskotch RW (1962) Tumor Inhibitors. i. aristolochic acid, the active principle of Aristolochia Indica. J Med Pharm Chem 91:657–659. https://doi.org/10.1021/jm01238a029
Brasche H (1968) Results with Tardolyt in poor surgical wound healing. Med Monatsschr 22:368–370
Filitis LN, Massagetov PS (1961) On the antineoplastic activity of aristolochic acid. Vopr Onkol 7(8):97–98
Jummel F (1964) On the efficacy of Aristolochic acid on chronic inflammatory tissue process. Ther Ggw 103:1020–1022
Moser H (1963) Clinical experience with aristolochic acid in earlier therapy-resistant prolonged suppurations. Langenbecks Archiv Fur Klinische Chir Vereinigt Mit Deutsche Z Fur Chir 304:660–663. https://doi.org/10.1007/BF02449160
Kluthe R, Vogt A, Batsford S (1982) Double blind study of the influence of aristolochic acid on granulocyte phagocytic activity. Arzneimittelforschung 32:443–445
Jackson L, Kofman S, Weiss A, Brodovsky H (1964) Aristolochic acid (NSC-50413): Phase I Clinical Study. Cancer Chemother Rep 42:35–37
Plestina R (1992) Some features of Balkan endemic nephropathy. Food Chem Toxicol 30:177–181. https://doi.org/10.1016/0278-6915(92)90030-o
Ceović S, Hrabar A, Sarić M (1992) Epidemiology of Balkan endemic nephropathy. Food Chem Toxicol 30:183–188. https://doi.org/10.1016/0278-6915(92)90031-f
Schmeiser HH, Pool BL, Wiessler M (1986) Identification and mutagenicity of metabolites of aristolochic acid formed by rat liver. Carcinogenesis 7:59–63. https://doi.org/10.1093/carcin/7.1.59
Vanherweghem JL, Depierreux M, Tielemans C, Abramowicz D, Dratwa M, Jadoul M, Richard C, Vandervelde D, Verbeelen D, Vanhaelen-Fastre R et al (1993) Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet 341:387–391. https://doi.org/10.1016/0140-6736(93)92984-2
Vanherweghem LJ (1998) Misuse of herbal remedies: the case of an outbreak of terminal renal failure in Belgium (Chinese herbs nephropathy). J Altern Complement Med 4:9–13. https://doi.org/10.1089/acm.1998.4.1-9
Pezzuto JM, Swanson SM, Mar W, Che CT, Cordell GA, Fong HH (1988) Evaluation of the mutagenic and cytostatic potential of aristolochic acid (3,4-methylenedioxy-8-methoxy-10-nitrophenanthrene-1-carboxylic acid) and several of its derivatives. Mutat Res 206:447–454. https://doi.org/10.1016/0165-1218(88)90052-3
Humans IWG, o t E o C R t, (2012) Pharmaceuticals. Volume 100 A. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 100:1–401
Michl J, Kite GC, Wanke S, Zierau O, Vollmer G, Neinhuis C, Simmonds MS, Heinrich M (2016) LC-MS- and (1)H NMR-based metabolomic analysis and in vitro toxicological assessment of 43 Aristolochia species. J Nat Prod 79:30–37. https://doi.org/10.1021/acs.jnatprod.5b00556
Michl J, Bello O, Kite GC, Simmonds MSJ, Heinrich M (2017) Medicinally used asarum species: high-resolution LC-MS analysis of aristolochic acid analogs and in vitro toxicity screening in HK-2 cells. Front Pharmacol 8:215. https://doi.org/10.3389/fphar.2017.00215
Grollman AP, Shibutani S, Moriya M, Miller F, Wu L, Moll U, Suzuki N, Fernandes A, Rosenquist T, Medverec Z, Jakovina K, Brdar B, Slade N, Turesky RJ, Goodenough AK, Rieger R, Vukelić M, Jelaković B (2007) Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci U S A 104:12129–12134. https://doi.org/10.1073/pnas.0701248104
Shibutani S, Bonala RR, Rosenquist T, Rieger R, Suzuki N, Johnson F, Miller F, Grollman AP (2010) Detoxification of aristolochic acid I by O-demethylation: less nephrotoxicity and genotoxicity of aristolochic acid Ia in rodents. Int J Cancer 127:1021–1027. https://doi.org/10.1002/ijc.25141
Wan J, Chen R, Yang Z, Xi J, Cao Y, Chen Y, Zhang X, Luan Y (2021) Aristolochic acid IVa forms DNA adducts in vitro but is non-genotoxic in vivo. Arch toxicol 95:2839–2850. https://doi.org/10.1007/s00204-021-03077-1
Xing G, Qi X, Chen M, Wu Y, Yao J, Gong L, Nohmi T, Luan Y, Ren J (2012) Comparison of the mutagenicity of aristolochic acid I and aristolochic acid II in the gpt delta transgenic mouse kidney. Mutat Res 743:52–58. https://doi.org/10.1016/j.mrgentox.2011.12.021
Xian Z, Tian J, Zhang Y, Meng J, Zhao Y, Li C, Yi Y, Han J, Liu S, Wang L, Pan C, Wang D, Wang F, Liang A (2021) Study on the potential nephrotoxicity and mutagenicity of aristolochic acid IVa and its mechanism. Biomed Pharmacother 142:112081. https://doi.org/10.1016/j.biopha.2021.112081
Sato N, Takahashi D, Chen SM, Tsuchiya R, Mukoyama T, Yamagata S, Ogawa M, Yoshida M, Kondo S, Satoh N, Ueda S (2004) Acute nephrotoxicity of aristolochic acids in mice. J Pharm Pharmacol 56:221–229. https://doi.org/10.1211/0022357023051
Liu S, Xian Z, Zhao Y, Wang L, Tian J, Pan C, Han J, Zhang Y, Li C, Yi Y, Liu C, Wang D, Meng J, Qin S, Wang F, Liang A (2021) Quantitative determination and toxicity evaluation of aristolochic acid analogues in Asarum heterotropoides F Schmidt (Xixin) and traditional chinese patent medicines. Front Pharmacol 12:761593. https://doi.org/10.3389/fphar.2021.761593
Hu X, Wang L, Zhang L, Zhang T (2020) β-Elemene inhibits 7,12-dimethylbenz(a)anthracene/12-O-tetradecanoylphorbol-13-acetate-induced skin tumorigenesis through suppression of NF-κB-associated signaling events in the mouse skin model. J Biochem Mol Toxicol 34:e22550. https://doi.org/10.1002/jbt.22550
Kulkarni NM, Muley MM, Jaji MS, Vijaykanth G, Raghul J, Reddy NK, Vishwakarma SL, Rajesh NB, Mookkan J, Krishnan UM, Narayanan S (2015) Topical atorvastatin ameliorates 12-O-tetradecanoylphorbol-13-acetate induced skin inflammation by reducing cutaneous cytokine levels and NF-κB activation. Arch Pharm Res 38:1238–1247. https://doi.org/10.1007/s12272-014-0496-0
Wu BC, Skovbakke SL, Masoudi H, Hancock REW, Franzyk H (2020) In vivo Anti-inflammatory Activity of Lipidated Peptidomimetics Pam-(Lys-βNspe)(6)-NH(2) and Lau-(Lys-βNspe)(6)-NH(2) Against PMA-Induced Acute Inflammation. Front Immunol 11:2102. https://doi.org/10.3389/fimmu.2020.02102
Park SH, Seo W, Eun HS, Kim SY, Jo E, Kim MH, Choi WM, Lee JH, Shim YR, Cui CH, Kim SC, Hwang CY, Jeong WI (2016) Protective effects of ginsenoside F2 on 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation in mice. Biochem Biophys Res Commun 478:1713–1719. https://doi.org/10.1016/j.bbrc.2016.09.009
Wang X, Shen Y, Wang S, Li S, Zhang W, Liu X, Lai L, Pei J, Li H (2017) PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucl Acids Res 45:W356-w360. https://doi.org/10.1093/nar/gkx374
Mendez D, Gaulton A, Bento AP, Chambers J, De Veij M, Félix E, Magariños MP, Mosquera JF, Mutowo P, Nowotka M, Gordillo-Marañón M, Hunter F, Junco L, Mugumbate G, Rodriguez-Lopez M, Atkinson F, Bosc N, Radoux CJ, Segura-Cabrera A, Hersey A, Leach AR (2019) ChEMBL: towards direct deposition of bioassay data. Nucl Acids Res 47:D930-d940. https://doi.org/10.1093/nar/gky1075
Das S, Thakur S, Korenjak M, Sidorenko VS, Chung FF, Zavadil J (2022) Aristolochic acid-associated cancers: a public health risk in need of global action. Nat Rev Cancer. https://doi.org/10.1038/s41568-022-00494-x
Mengs U (1988) Tumour induction in mice following exposure to aristolochic acid. Arch toxicol 61:504–505. https://doi.org/10.1007/bf00293699
Schmeiser HH, Schoepe KB, Wiessler M (1988) DNA adduct formation of aristolochic acid I and II in vitro and in vivo. Carcinogenesis 9:297–303. https://doi.org/10.1093/carcin/9.2.297
Mengs U, Stotzem CD (1993) Renal toxicity of aristolochic acid in rats as an example of nephrotoxicity testing in routine toxicology. Arch toxicol 67:307–311. https://doi.org/10.1007/bf01973700
Debelle FD, Vanherweghem JL, Nortier JL (2008) Aristolochic acid nephropathy: a worldwide problem. Kidney Int 74:158–169. https://doi.org/10.1038/ki.2008.129
Rosenthal MD, Sannanaik Vishwanath B, Franson RC (1989) Effects of aristolochic acid on phospholipase A2 activity and arachidonate metabolism of human neutrophils. Biochim Et Biophys Et Biophys Acta 1001:1–8. https://doi.org/10.1016/0005-2760(89)90299-3
Moreno JJ (1993) Effect of aristolochic acid on arachidonic acid cascade and in vivo models of inflammation. Immunopharmacology 26:1–9. https://doi.org/10.1016/0162-3109(93)90061-T
Liu MC, Lin TH, Wu TS, Yu FY, Lu CC, Liu BH (2011) Aristolochic acid I suppressed iNOS gene expression and NF-κB activation in stimulated macrophage cells. Toxicol Lett 202:93–99. https://doi.org/10.1016/j.toxlet.2011.01.021
Gad SC (1994) The mouse ear swelling test (MEST) in the 1990s. Toxicology 93:33–46. https://doi.org/10.1016/0300-483x(94)90194-5
Kim SY, Son KH, Chang HW, Kang SS, Kim HP (1999) Inhibition of mouse ear edema by steroidal and triterpenoid saponins. Arch Pharm Res 22:313–316. https://doi.org/10.1007/bf02976370
Han NR, Ko SG, Moon PD, Park HJ (2021) Chloroquine attenuates thymic stromal lymphopoietin production via suppressing caspase-1 signaling in mast cells. Biomed Pharmacother 141:111835. https://doi.org/10.1016/j.biopha.2021.111835
Zelová H, Hošek J (2013) TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm Res 62:641–651. https://doi.org/10.1007/s00011-013-0633-0
Czock D, Keller F, Rasche FM, Häussler U (2005) Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 44:61–98. https://doi.org/10.2165/00003088-200544010-00003
Macía MA, Frias J, Carcas AJ, Guerra P, Valiente R, Lucero ML (1995) Comparative bioavailability of a dispersible formulation of diclofenac and finding of double plasma peaks. Int J Clin Pharmacol Ther 33:333–339
Davies NM, Anderson KE (1997) Clinical pharmacokinetics of diclofenac Therapeutic insights and pitfalls. Clin Pharmacokinet 33:184–213. https://doi.org/10.2165/00003088-199733030-00003
Capone ML, Sciulli MG, Tacconelli S, Grana M, Ricciotti E, Renda G, Di Gregorio P, Merciaro G, Patrignani P (2005) Pharmacodynamic interaction of naproxen with low-dose aspirin in healthy subjects. J Am Coll Cardiol 45:1295–1301. https://doi.org/10.1016/j.jacc.2005.01.045
Vishwanath BS, Appu Rao AG, Gowda TV (1987) Interaction of phospholipase A2 from Vipera russelli venom with aristolochic acid: a circular dichroism study. Toxicon 25:939–946. https://doi.org/10.1016/0041-0101(87)90156-5
Vishwanath BS, Gowda TV (1987) Interaction of aristolochic acid with Vipera russelli phospholipase A2: its effect on enzymatic and pathological activities. Toxicon 25:929–937. https://doi.org/10.1016/0041-0101(87)90155-3
Chandra V, Jasti J, Kaur P, Srinivasan A, Betzel C, Singh TP (2002) Structural basis of phospholipase A2 inhibition for the synthesis of prostaglandins by the plant alkaloid aristolochic acid from a 1.7 A crystal structure. Biochemistry 41:10914–10919. https://doi.org/10.1021/bi0258593
Vishwanath BS, Fawzy AA, Franson RC (1988) Edema-inducing activity of phospholipase A2 purified from human synovial fluid and inhibition by aristolochic acid. Inflammation 12:549–561. https://doi.org/10.1007/bf00914317
Sborchia M, Keun HC, Phillips DH, Arlt VM (2019) The impact of p53 on aristolochic acid i-induced gene expression in vivo. Int J Mol Sci. https://doi.org/10.3390/ijms20246155
Stachurska A, Kozakowska M, Jozkowicz A, Dulak J, Loboda A (2011) Aristolochic acid I and ochratoxin A differentially regulate VEGF expression in porcine kidney epithelial cells–the involvement of SP-1 and HIFs transcription factors. Toxicol Lett 204:118–126. https://doi.org/10.1016/j.toxlet.2011.04.022
Bradford KL, Moretti FA, Carbonaro-Sarracino DA, Gaspar HB, Kohn DB (2017) Adenosine Deaminase (ADA)-Deficient Severe Combined Immune Deficiency (SCID): molecular pathogenesis and clinical manifestations. J Clin Immunol 37:626–637. https://doi.org/10.1007/s10875-017-0433-3
Gao ZW, Wang X, Zhang HZ, Lin F, Liu C, Dong K (2021) The roles of adenosine deaminase in autoimmune diseases. Autoimmun Rev 20:102709. https://doi.org/10.1016/j.autrev.2020.102709
Sun C, Wang B, Hao S (2022) Adenosine-A2A receptor pathway in cancer immunotherapy. Front Immunol 13:837230. https://doi.org/10.3389/fimmu.2022.837230
Ross JS, Fletcher JA (1999) HER-2/neu (c-erb-B2) gene and protein in breast cancer. Am J Clin Pathol 112:S53-67
Waks AG, Winer EP (2019) Breast cancer treatment: a review. JAMA 321:288–300. https://doi.org/10.1001/jama.2018.19323
Hegde VR, Borges S, Pu H, Patel M, Gullo VP, Wu B, Kirschmeier P, Williams MJ, Madison V, Fischmann T, Chan TM (2010) Semi-synthetic aristolactams–inhibitors of CDK2 enzyme. Bioorg Med Chem Lett 20:1384–1387. https://doi.org/10.1016/j.bmcl.2010.01.007
Hsu AY, Wang D, Liu S, Lu J, Syahirah R, Bennin DA, Huttenlocher A, Umulis DM, Wan J, Deng Q (2019) Phenotypical microRNA screen reveals a noncanonical role of CDK2 in regulating neutrophil migration. Proc Natl Acad Sci U S A 116:18561–18570. https://doi.org/10.1073/pnas.1905221116
Lichtman MK, Otero-Vinas M, Falanga V (2016) Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen 24:215–222. https://doi.org/10.1111/wrr.12398
Acknowledgements
We would like to thank Professor. Xin Dong for his constructive suggestions. We appreciate Dr. Yinzhong Lu, Dr. Bo Han, and Mu Wang for their help in experiments. We thank Dr. Tianpei Xie and Dr. Yong Qian from Shanghai Standard Technology Co., Ltd. for their helpful discussions and support on AAIVa.
Funding
This work is sponsored by National Natural Science Foundation of China (Grant number 81873081), Foundation of Shanghai Municipal Heath Commission (grant number 202040009) and Foundation of Science and Technology Commission of Shanghai Municipality (grant number 20142202700, 16DZ2280800).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethical approval
All animal experiments were approved by The Animal Care and Welfare Committee of Shanghai Jiao Tong University School of Medicine (A-2019–001), Shanghai, China. No clinical studies or patient data are contained in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, W., Shao, F., You, X. et al. Non-carcinogenic/non-nephrotoxic aristolochic acid IVa exhibited anti-inflammatory activities in mice. J Nat Med 77, 251–261 (2023). https://doi.org/10.1007/s11418-022-01665-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-022-01665-8