Abstract
The aging population worldwide is facing a significant increase in age-related non-communicable diseases, including cardiovascular and brain pathologies. This comprehensive review paper delves into the impact of the exposome, which encompasses the totality of environmental exposures, on unhealthy aging. It explores how environmental factors contribute to the acceleration of aging processes, increase biological age, and facilitate the development and progression of a wide range of age-associated diseases. The impact of environmental factors on cognitive health and the development of chronic age-related diseases affecting the cardiovascular system and central nervous system is discussed, with a specific focus on Alzheimer’s disease, Parkinson’s disease, stroke, small vessel disease, and vascular cognitive impairment (VCI). Aging is a major risk factor for these diseases. Their pathogenesis involves cellular and molecular mechanisms of aging such as increased oxidative stress, impaired mitochondrial function, DNA damage, and inflammation and is influenced by environmental factors. Environmental toxicants, including ambient particulate matter, pesticides, heavy metals, and organic solvents, have been identified as significant contributors to cardiovascular and brain aging disorders. These toxicants can inflict both macro- and microvascular damage and many of them can also cross the blood–brain barrier, inducing neurotoxic effects, neuroinflammation, and neuronal dysfunction. In conclusion, environmental factors play a critical role in modulating cardiovascular and brain aging. A deeper understanding of how environmental toxicants exacerbate aging processes and contribute to the pathogenesis of neurodegenerative diseases, VCI, and dementia is crucial for the development of preventive strategies and interventions to promote cardiovascular, cerebrovascular, and brain health. By mitigating exposure to harmful environmental factors and promoting healthy aging, we can strive to reduce the burden of age-related cardiovascular and brain pathologies in the aging population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global population is experiencing a significant increase in the number and proportion of individuals aged 60 years and older. According to the World Health Organization (WHO), the number of people over 60 years old reached 1 billion in 2019 and is projected to surpass 2.1 billion by 2050 [1]. This demographic shift is accompanied by a rising prevalence of age-related non-communicable diseases (NCDs) in aging societies, particularly in the Western world. These NCDs include cardiovascular and cerebrovascular diseases (such as heart failure, myocardial infarction, stroke, vascular cognitive impairment), cancers, chronic respiratory diseases (like chronic obstructive pulmonary disease), chronic kidney disease, type-2 diabetes mellitus, musculoskeletal diseases, and neurodegenerative diseases. The impact of these chronic age-related NCDs on the quality of life for affected individuals spans several decades and carries substantial socioeconomic implications for Western societies. Deterioration of cognitive health associated with age-related NCDs is especially important in that regard.
In the USA, over 90% of older individuals have at least one chronic NCD, with approximately three-quarters experiencing two or more [2]. The economic burden associated with age-related chronic diseases has been estimated at a staggering $47 trillion for the period from 2010 to 2030 [3]. The financial strain imposed by the costly care required for older individuals with age-related NCDs affects pension systems and healthcare systems alike. Recognizing the magnitude of this problem, the World Health Organization emphasizes the importance of focusing on prevention of age-related NCDs and the promotion of healthy aging [4, 5].
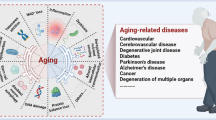
In recent years, advances in geroscience have led to a paradigm shift in our understanding of the pathogenesis of chronic age-related NCDs [6, 7]. It is now recognized that all age-associated diseases share common underlying cellular and molecular mechanisms of aging. These mechanisms include increased oxidative stress, cellular mitochondrial and energetic dysfunction, impaired cellular stress resilience, genetic instability and DNA damage, induction of cell senescence, heightened state of inflammation, epigenetic dysregulation, altered proteostasis, disruption of intercellular communication (including endocrine changes), stem cell dysfunction, and dysregulation of energy sensing pathways [6, 8,9,10] (Fig. 1). These pathways are genetically determined, but environmental and lifestyle factors play a critical role in modulating the rate of cellular and organismal aging, thereby defining the trajectories of age-related functional decline (Fig. 1). It is now emerging that environmental and lifestyle risk factors can exacerbate fundamental cellular and molecular aging processes, promoting accelerated aging phenotypes and the premature development of chronic age-related NCDs (Fig. 1).
Conceptual model illustrating the contribution of environmental drivers to unhealthy aging, characterized by accelerated aging processes, increased biological age, and the development and progression of various age-associated diseases. Environmental toxicants (depicted in orange) play a key role in promoting age-related cardiovascular, cerebrovascular, and brain pathologies, as well as the pathogenesis of age-associated diseases in other organ systems. These toxicants exacerbate fundamental molecular and cellular aging processes (depicted as roots), which serve as the underlying mechanisms. The consequences of accelerated vascular aging induced by toxicants give rise to the genesis of micro- and macrovascular pathologies, including atherosclerotic vascular diseases, cerebral small vessel disease, and Alzheimer’s disease. Furthermore, many of these toxicants have the ability to cross the blood–brain barrier, leading to neurotoxic effects, neuroinflammation, and neuronal dysfunction, promoting the genesis of neurodegenerative diseases and cognitive decline. Clinical disciplines, biogerontology, and environmental toxicology, along with public health research, traditionally focus on specific age-related diseases (depicted as leaves), mechanisms of aging (depicted as roots), and environmental risk factors, respectively. Geroscience, as an integrative scientific field, considers the interaction between all these levels, facilitating a comprehensive understanding of the complex relationship between environmental factors and unhealthy aging
The role of the environment in controlling human aging is increasingly being explored within an exposomic framework [11]. The exposome, as originally proposed by Dr. Wild in 2005 [12], encompasses all environmental exposures from conception that influence health outcomes. It encompasses internal factors (e.g., physical activity, microbiome, metabolism), general external factors (e.g., education, social status, climate, urban–rural environment), and specific external factors (environmental pollutants and chemical contaminants, lifestyle factors, occupation). It is worth noting that in our developed world, the majority of the population is exposed to approximately 80,000 to 100,000 artificially produced substances in their daily lives. In heavily polluted work environments, this number can reach up to 200,000 substances. The estimated number of “manmade” xenobiotic compounds currently stands at 10 million, highlighting the extensive exposure to synthetic chemicals in our modern society. These environmental toxicants have the potential to disrupt physiological processes and promote the pathogenesis of NCDs. Understanding the impact of this vast array of chemical exposures on aging and age-related diseases is critical for public health. Studies employing environmental epidemiology approaches, including geographical data linkages, have confirmed the significant contribution of exposure to environmental pollutants, including particulate matter in air pollution and occupational exposures, to the shared exposome, influencing longevity, geographic clustering, and the development of various age-related chronic NCDs such as neurodegenerative diseases, cardiovascular diseases, and cancer [11, 13,14,15,16,17,18,19,20,21,22,23].
Notably, exposure to environmental toxicants has been shown to associate with molecular hallmarks of aging [24,25,26,27,28,29,30,31]. In this review, we delve into the pathophysiological roles of environmental toxicants in modulating the fundamental cellular and molecular mechanisms of aging. Specifically, we examine their contributions to the pathogenesis of age-related NCDs, with a particular focus on cardiovascular, cerebrovascular and brain pathologies (Fig. 1). The selection of exposure factors considered in this review is based on an extensive literature search, encompassing a wide range of environmental toxicants, including ambient particulate matter, pesticides, heavy metals, and organic solvents, which have been previously identified as significant contributors to aging disorders in both the cardiovascular system and the brain. By exploring the interconnectedness between potential mechanisms of cardiovascular, cerebrovascular, and brain aging and the pathways affected by environmental factors, we gain insights into the complex relationships between environmental toxicants and unhealthy aging processes. Additionally, we explore the interaction between these cellular and molecular aging processes and disease-specific pathways. By examining the intricate relationships between environmental toxicants and cardiovascular, cerebrovascular, and brain aging, we can identify potential targets for public health interventions aimed at promoting healthy aging. By implementing preventive measures and interventions to mitigate the detrimental effects of environmental toxicants, we can strive to improve the overall well-being of the aging population. Our comprehensive review aims to provide valuable insights into the multifaceted role of environmental drivers in aging and guide future research efforts and public health strategies for healthy aging.
Exacerbation of chronic age-related diseases by environmental toxicants
From a geroscience perspective, certain “risk factors” contribute to the pathogenesis of NCDs by exacerbating cellular and molecular mechanisms of aging (Fig. 1). Among these factors, environmental toxicants have been found to have significant detrimental effects on biological aging processes [20, 23, 24, 26,27,28,29,30,31,32,33,34,35]. Research indicates that environmental toxicants play a critical role in the development of NCDs, with the World Health Organization (WHO) estimating that 24% (13.7 million) of all global deaths per year are linked to environmental factors [36]. Within this number, 8.5 million deaths are attributed to NCDs, and the top three causes related to the environment are ischemic heart disease, chronic respiratory diseases, and cancer. In the following sections, we will briefly discuss the interaction between environmental toxicants, aging processes, and the genesis of organ-specific NCDs.
Cardiovascular diseases
Vascular aging contributes to the age-dependent rise in a broad range of macrovascular and microvascular pathologies, including hypertension, atherosclerotic diseases (such as ischemic heart disease, peripheral artery disease, and stroke), aortic aneurysms, heart failure, cerebral small vessel disease, and age-related microvascular pathologies affecting other organs (such as glomerulosclerosis, microvascular rarefaction, and retinal pathologies) [8, 37, 38]. Aging alone confers a significantly higher risk for these diseases compared to “conventional” risk factors like lipid levels, smoking, diabetes mellitus, and sedentary lifestyle [8, 37].
The cellular and molecular mechanisms of aging that contribute to the pathogenesis of age-related cardiovascular diseases have been the subject of recent reviews [8, 37, 39]. Increased oxidative stress plays a significant role in vascular aging [8, 37, 40,41,42,43,44,45,46,47,48,49,50,51]. The elevated production of reactive oxygen species (ROS) leads to endothelial dysfunction by inactivating endothelium-derived nitric oxide (NO) and producing peroxynitrite (ONOO-) [8, 37, 52]. Consequently, it results in age-related reduction in endothelium-dependent dilation [53, 54], enhanced vasoconstriction, and dysregulation of tissue perfusion [8, 37]. The lack of NO and increased oxidative stress also promotes vascular inflammation and the development of a proatherogenic vascular phenotype in aging [8, 37, 50]. Increased oxidative stress can activate matrix metalloproteinases, which disrupt the structural integrity of aged arteries, potentially contributing to large artery stiffening and the pathogenesis of aortic aneurysms and cerebral microhemorrhages [37, 44, 49, 55,56,57,58,59]. Oxidative stress is also associated with DNA damage and DNA damage–induced cellular senescence, which are additional mechanisms of aging contributing to vascular pathologies [37, 60, 61].
Mitochondrial alterations, including mitochondrial ROS production, impaired mitochondrial biogenesis, impaired cellular energy production, and mitochondrial DNA damage, further exacerbate vascular aging processes [8, 37, 39, 62,63,64,65,66,67,68,69]. Additionally, the release of several proinflammatory molecules increases with aging, leading to macrovascular and microvascular pathologies such as atherogenesis, aneurysm formation, and microvascular dysfunction [37].
The role of Nrf2, which coordinates an adaptive antioxidant response, has also been emphasized in recent studies [70,71,72,73,74,75,76,77,78,79,80]. Aging causes Nrf2 dysfunction in the vasculature, impairing the oxidative stress resilience of aged cells and contributing to age-related vascular pathologies induced by pro-oxidative, DNA damaging stimuli [75,76,77]. Aging also impacts the proteostasis system of the vasculature by impairing chaperones, the ubiquitin–proteasome system, and the lysosome-autophagy system [8, 37]. Furthermore, endothelial senescence contributes to endothelial dysfunction in aging and pathophysiological conditions associated with accelerated vascular aging [8, 37, 46, 73, 81,82,83]. Epigenetic alterations, such as changes in DNA methylation patterns or miRNA dysregulation, also contribute to impairment of angiogenic processes, vascular inflammation, or atherogenesis [8, 37].
The heart and vascular system are highly vulnerable to various environmental agents. The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) estimated that long-term exposure to ambient fine particulate matter (PM2.5) contributed to 2.9 million deaths globally each year, with nearly 50% of these deaths attributable to ischemic heart disease and stroke [84]. Even at concentrations below current regulatory standards in the USA and European Union, long-term exposure to PM10 and PM2.5 within metropolitan areas is associated with the progression of coronary calcification, indicating accelerated atherosclerosis [85]. Air pollution has been identified as a risk factor for ischemic heart disease [86] and stroke [87, 88]. Ambient air pollution and household air pollution from cooking with polluting fuels are estimated to cause 13 and 17% of cardiovascular diseases, respectively [89,90,91]. Acrolein, a highly reactive unsaturated aldehyde, is generated during the burning of diesel fuels, gasoline, woods, plastics, cigarette smoking, and frying of food with fats. The Environmental Protection Agency classifies acrolein as a high-priority air and water toxicant. Strong evidence suggests that acrolein can cause damage to cardiac myocytes and endothelial cells, promoting endothelial dysfunction, vascular disease, and heart failure [92,93,94,95].
Smoking and exposure to secondhand smoke play significant roles in the pathogenesis of cardiovascular diseases [96, 97]. Cigarette smoke contains numerous harmful substances, including carbon monoxide, nicotine, and a range of toxic chemicals and particulate matter. Smoking is a well-established risk factor for cardiovascular diseases, particularly ischemic heart disease, peripheral artery disease, stroke and vascular cognitive impairment [96, 97]. The detrimental effects of smoking on the cardiovascular system arise from multiple mechanisms [98,99,100,101,102,103,104,105,106,107,108,109]. Firstly, smoking promotes the development of atherosclerosis by damaging the endothelial lining of blood vessels, increasing inflammation, and accelerating the formation of fatty deposits within the arterial walls [96, 97]. Secondly, smoking leads to increased oxidative stress and endothelial dysfunction, impairing the production and bioavailability of NO and promoting nitrative stress [96, 97, 103, 110, 111]. Increased oxidative stress also contributes to lipid oxidation, induces expression of adhesion molecules of the endothelium and activates macrophages and platelets [96, 97, 110, 111]. Macrophages engulf oxidized lipids, leading to the formation of foam cells within the aortic wall. Subsequent foam cell death triggers the release of these lipids, promoting the development of lipid-rich aortic plaques [96]. Furthermore, cigarette smoke leads to an increased expression and activity of matrix metalloproteinases (MMPs), as well as to decreased expression of MMP inhibitors causing alterations of vascular extracellular matrix and tissue remodeling that play a significant role not just in atherogenesis but in the formation of aneurysms as well [96]. Moreover, smoking promotes platelet aggregation and blood clot formation, increasing the risk of thrombotic events. Additionally, smoking causes an unfavorable lipid profile, with lower levels of beneficial high-density lipoprotein (HDL) cholesterol and elevated levels of harmful low-density lipoprotein (LDL) cholesterol. Furthermore, smoking induces systemic inflammation and adversely affects the balance of various pro-inflammatory and anti-inflammatory molecules, contributing to the progression of cardiovascular diseases [98, 102, 103, 112,113,114]. Results from the Cardiovascular Health Study suggest that smoking-induced inflammation promotes cardiomyocyte injury exacerbating heart failure [115]. Secondhand smoke, which is the involuntary inhalation of smoke emitted by smokers, also poses a significant risk to cardiovascular health. Exposure to secondhand smoke has been associated with an increased risk of developing cardiovascular diseases, similar to active smoking. The toxic components in secondhand smoke can lead to endothelial dysfunction, inflammation, and increased oxidative stress in non-smokers, thereby contributing to the pathogenesis of cardiovascular diseases. Overall, the avoidance of smoking and secondhand smoke exposure is crucial for preventing and reducing the burden of cardiovascular diseases.
Water pollution is a significant environmental concern that can have adverse effects on cardiovascular health. Various pollutants and contaminants found in water sources can increase the risk of cardiovascular diseases [116]. For example, arsenic in drinking water has been identified as a potential risk factor for cardiovascular diseases [117]. Prolonged exposure to elevated levels of arsenic in drinking water has been associated with an increased incidence of hypertension, atherosclerosis, and cardiovascular mortality [117]. Similarly, exposure to lead and cadmium, often found in contaminated water sources, has been linked to an elevated risk of developing cardiovascular diseases [118,119,120,121,122,123,124,125,126]. Other pollutants present in water pollution that can pose harm to the cardiovascular system include mercury. Mercury is a toxic heavy metal that can contaminate water sources, primarily through industrial processes and the burning of fossil fuels. Chronic exposure to mercury has been associated with an increased risk of cardiovascular diseases, including hypertension, coronary artery disease, and myocardial infarction [127,128,129,130,131,132,133,134]. Water pollution may also contain various organic pollutants, such as polycyclic aromatic hydrocarbons (PAHs), pesticides, and industrial chemicals. These substances have been linked to adverse cardiovascular effects, including endothelial dysfunction, oxidative stress, inflammation, and disruption of cardiac function [135,136,137]. Water treatment processes often involve the use of chlorine and other disinfectants to eliminate microbial contaminants. However, the reaction between chlorine and organic matter in water can lead to the formation of disinfection by-products (DBPs), such as trihalomethanes (THMs) and haloacetic acids (HAAs) [138,139,140]. Long-term exposure to elevated levels of DBPs in drinking water has been associated with an increased risk of cardiovascular diseases, particularly in relation to heart disease and adverse cardiac remodeling [138,139,140]. Microplastics are tiny plastic particles that have become a pervasive environmental pollutant, including in water sources. While the direct impact of microplastics on cardiovascular health is still being studied, emerging evidence suggests that microplastic exposure may contribute to oxidative stress, inflammation, and endothelial dysfunction, all of which can increase the risk of cardiovascular diseases [141,142,143,144,145,146,147]. The specific health effects of water pollution may vary depending on the concentration and duration of exposure. To safeguard cardiovascular health, ensuring access to clean and uncontaminated water sources is crucial, along with implementing effective water treatment and pollution control measures.
The pollutants mentioned, ranging from PM2.5 and cigarette smoke to microplastics, can exacerbate biological mechanisms of aging through various interconnected pathways. Firstly, these pollutants contribute to increased oxidative stress within cells of the cardiovascular system and other organs [148,149,150,151,152,153,154,155,156,157,158]. They generate excessive reactive oxygen species (ROS), overwhelming the body’s antioxidant defense mechanisms and leading to oxidative damage to cellular structures, including DNA, proteins, and lipids. This oxidative stress contributes to the acceleration of aging processes, as well as the development of age-related diseases.
Secondly, exposure to these pollutants can induce increased DNA damage and cellular senescence [159,160,161,162,163]. DNA damage can occur due to direct interaction with the genetic material or through the generation of ROS. Persistent exposure to pollutants can lead to accumulation of DNA damage, impairing the cell’s ability to repair and maintain genomic integrity. In turn, this initiates cellular senescence, a state of irreversible growth arrest characterized by significant phenotypic alterations, including the emergence of the pro-inflammatory senescence-associated secretory phenotype (SASP). This senescence-driven inflammatory milieu contributes to tissue dysfunction and accelerates the aging process.
Furthermore, pollutants have been shown to impact stem cell function [164,165,166,167,168,169,170,171,172,173,174]. Pesticides, tobacco smoke, and heavy metals have been identified as disruptors of stem cell homeostasis, leading to impaired regenerative capacity and tissue repair. This disruption can further contribute to accelerated aging and compromised organ function.
Inflammation is another critical mechanism affected by these pollutants [10, 155, 175,176,177,178,179,180,181,182,183,184,185,186]. Chronic exposure to pollutants can trigger a sustained inflammatory response within the cardiovascular system and other organs as well. Inflammatory molecules are released, leading to the activation of immune cells and the production of pro-inflammatory mediators. This chronic state of inflammation contributes to tissue damage, promotes aging-related pathologies, and increases the risk of age-related diseases.
Additionally, pollutants can disrupt mitochondrial function, leading to mitochondrial dysfunction [24, 26, 27, 30]. These toxic substances interfere with mitochondrial processes, such as energy production and oxidative phosphorylation. As a consequence, mitochondrial dysfunction occurs, leading to decreased energy availability, increased oxidative stress, and compromised cellular function.
In summary, environmental pollutants exacerbate biological mechanisms of aging through increased oxidative stress, increased DNA damage and cellular senescence, stem cell dysfunction, inflammation, mitochondrial dysfunction, and other interconnected pathways. Pollutants can also alter epigenetic mechanisms of aging [187]. Understanding these mechanisms is crucial for developing strategies to mitigate the detrimental effects of pollutants and promote healthy aging.
Environmental drivers of unhealthy cerebrovascular and brain aging
Alzheimer’s disease and Parkinson’s disease are the two most prevalent age-related neurodegenerative diseases affecting the central nervous system. These conditions have a profound impact on cognitive function, motor control, and overall quality of life, particularly in the elderly population. As individuals age, the incidence of both Alzheimer’s and Parkinson’s diseases increases, highlighting the significant burden these conditions pose on global health [188,189,190]. The pathogenesis of Alzheimer’s and Parkinson’s diseases involves a complex interplay of genetic, environmental, and lifestyle factors. While the exact causes of these diseases remain to be fully elucidated, several mechanisms have been proposed to contribute to their development and progression. In the case of Alzheimer’s disease, important hallmarks are microvascular pathologies (amyloid angiopathy, microhemorrhages, blood–brain barrier disruption) [191,192,193,194,195,196,197,198,199,200,201] and accumulation of amyloid-beta plaques and tau tangles in the brain. These abnormal protein aggregates disrupt normal neuronal communication and function, leading to cognitive decline. Importantly, hypertension has been found to exacerbate several manifestations of Alzheimer’s disease [55, 202,203,204,205]. Aging itself plays a crucial role in the development of Alzheimer’s disease by contributing to increased oxidative stress [148, 206, 207], impaired mitochondrial function [25], DNA damage [208,209,210,211], and inflammation [212]. These aging-related processes can have significant implications for the cerebral microcirculation, leading to the emergence of microvascular pathologies and promoting the formation and accumulation of amyloid-beta and tau pathology, the hallmark features of Alzheimer’s disease. Additionally, genetic factors, such as mutations in genes like amyloid precursor protein (APP) and presenilin 1 and 2 (PSEN1 and PSEN2), can further increase the risk of developing Alzheimer’s disease.
Parkinson’s disease, on the other hand, is characterized by the degeneration of dopaminergic neurons in the substantia nigra region of the brain. This neuronal loss leads to motor symptoms such as tremors, rigidity, and bradykinesia. Aging is a significant risk factor for Parkinson’s disease, as the brain undergoes age-related changes that contribute to the vulnerability of dopaminergic neurons. These changes include mitochondrial dysfunction, impaired protein handling and clearance mechanisms, oxidative stress, and inflammation. Additionally, genetic factors, such as mutations in the alpha-synuclein (SNCA) gene and genes involved in mitochondrial function, can increase the susceptibility to Parkinson’s disease.
Cognitive impairment caused by macrovascular (atherosclerosis) and microvascular pathologies (vascular cognitive impairment or VCI) is the second most common form of age-related cognitive decline [213,214,215,216,217]. Microvascular pathologies also play a central role in the pathogenesis of Alzheimer’s disease [55, 191, 193, 194, 198, 218, 219]. Age-related changes in the microvasculature [220,221,222,223,224,225], including alterations in endothelial function [59, 226, 227], blood–brain barrier integrity [199, 200, 228], and cerebral blood flow regulation, contribute to cognitive impairment and the development of neurodegenerative diseases.
Environmental toxicants can adversely affect cerebrovascular and brain health. Common sources of environmental pollutants linked to neurotoxic manifestations include pesticides, solvents, industrial waste, automobile exhaust, cigarette smoke and burning of terrestrial waste. Growing epidemiological and experimental evidence suggests that exposure to environmental toxicants, such as pesticides [229], heavy metals (e.g., lead) and organic solvents (e.g., trichloroethylene, n-hexane, and others) [230, 231], exerts neurotoxic effects and may increase the risk of developing Alzheimer’s and Parkinson’s diseases [25]. These toxicants may damage the cerebral microvasculature and also can cross the blood–brain barrier, leading to cytotoxic effects, neuroinflammation, and consequential neuronal dysfunction and injury. Ambient outdoor air pollution has also been implicated in the exacerbation of the pathogenesis of both Parkinson’s and Alzheimer’s diseases [148, 208, 232,233,234,235,236,237,238,239,240,241,242]. Longitudinal cohort studies have shown associations between increased levels of PM2.5 (fine particulate matter) and a higher hazard of hospital admission for Parkinson’s disease and Alzheimer’s disease and related dementias [243]. Neurovascular damage and cerebromicrovascular dysfunction are increasingly recognized as important contributors to cognitive decline and neurodegeneration [59, 199,200,201, 244,245,246]. Cells of the neurovascular unit, including cerebromicrovascular endothelial cells, pericytes, astrocytes, and perivascular microglia, are sensitive to the harmful effects of environmental toxicants [29, 247, 248].
The cellular and molecular mechanisms underlying the impact of environmental toxicants on neurodegeneration and neurovascular injury can involve mitochondrial dysfunction [25,26,27,28,29,30,31], which impairs cellular energy production, metabolism, alters intracellular signaling, promotes increased free radical production, and apoptosis. Environmental toxicants can also exacerbate oxidative stress [208], which is causally linked to microglia activation and neuroinflammation [10], cellular senescence [249, 250], and protein aggregation, ultimately leading to neuronal damage and death. It is likely that air pollution contributes to neuronal injury, oxidative stress, neuroinflammation, and cerebromicrovascular impairment, thereby exacerbating the pathogenesis of neurodegenerative diseases [148, 208, 235, 237, 241, 242]. The complex interplay between environmental toxicants and the underlying cellular and molecular mechanisms highlights the importance of understanding and mitigating the impact of these environmental drivers to promote brain health and reduce the burden of cerebrovascular and neurodegenerative disorders.
Pulmonary diseases
Aging has a significant impact on the incidence and development of pulmonary diseases, particularly chronic obstructive pulmonary disease (COPD [251,252,253,254]) and other respiratory disorders. In 2019, there were 212.3 million prevalent cases of COPD reported globally, resulting in 3.3 million deaths and accounting for 74.4 million DALYs (disability-adjusted life years) [255]. The prevalence and death rate of COPD show an increasing tendency with age, peaking in the oldest age group (≥ 95 years) [255]. Studies have revealed that the prevalence of COPD is two to three times higher in individuals over the age of 60 years compared to younger age groups [256]. Furthermore, there are striking similarities between the mechanisms of lung aging and COPD, including cell senescence, shortened telomeres, inflammation, and oxidative stress, suggesting that accelerated aging processes may be involved in the pathogenesis of COPD [256,257,258,259].
Ambient particulate matter, a major component of outdoor air pollution, is considered a significant risk factor for respiratory disorders such as COPD. While tobacco smoking was traditionally seen as the primary cause of COPD, it is now recognized that air pollution, including fine particulate matter, plays a substantial role in the development and progression of the disease. According to the World Health Organization (WHO), 18% of premature deaths related to outdoor air pollution are attributed to COPD, making it the most prevalent chronic respiratory disorder [260]. Additionally, 25% of deaths from chronic COPD can be attributed to exposure to household air pollution, primarily in low- and middle-income countries. Certain occupational environments, such as coal and hard-rock mining, construction work, and various manufacturing industries (e.g., concrete, plastics, textiles, rubber, leather, and food products), pose a high risk for COPD [261,262,263].
The cellular and molecular mechanisms underlying the impact of air pollution and other environmental toxicants on the genesis of pulmonary diseases involve several interconnected pathways. These include increased oxidative stress, DNA damage, cellular senescence, inflammation, and mitochondrial dysfunction. Exposure to air pollution and environmental toxicants leads to an imbalance between the production and neutralization of ROS, resulting in increased oxidative stress. This oxidative stress contributes to cellular injury, DNA damage and induction of senescence, inflammation, and the activation of signaling pathways involved in the pathogenesis of pulmonary diseases. Chronic inflammation, triggered by environmental toxicants, can perpetuate tissue damage and contribute to the progression of pulmonary diseases. Additionally, mitochondrial dysfunction, caused by exposure to pollutants, disrupts cellular energy production and metabolism, further compromising lung function.
Malignant diseases
The etiology of cancer is multifaceted, influenced by a wide range of factors, and varies depending on the specific type of cancer. Many cancers are considered quintessential diseases of aging (e.g., colorectal cancer, multiple myeloma [264]), as their incidence increases exponentially with age, and mechanisms associated with aging contribute to their pathogenesis [265, 266]. In animal models, interventions and genetic manipulations that delay aging and extend lifespan, such as caloric restriction, have been shown to exert significant anti-cancer effects [267,268,269,270,271,272,273,274]. Conversely, interventions and genetic manipulations that accelerate aging and shorten lifespan promote tumorigenesis and cancer progression [275,276,277].
Numerous environmental factors have been causally linked to the genesis of various cancer types. Outdoor air pollution, for example, has been identified as a cause of lung cancer occurrence [278, 279] and is increasingly being associated with other types of cancer, including bladder cancer and breast cancer [280]. Indoor air pollution resulting from the burning of solid fuels has been associated with oral, cervical, and esophageal cancer [279].
Workplace or home exposure to a wide range of chemicals has also been causally linked to the development of diverse types of cancer. Asbestos, silica, diesel exhaust, uranium, arsenic, beryllium, cadmium, silica, vinyl chloride, nickel compounds, chromium compounds, coal products, mustard gas, and chloromethyl ethers are significant risk factors for lung cancer [281,282,283,284,285,286,287,288,289,290,291]. Exposure to vinyl chloride increases the risk of liver cancer [292,293,294,295], while limited evidence suggests an increased risk with exposure to arsenic [296] and trichloroethylene [297, 298].
The International Agency for Research on Cancer (IARC) classifies asbestos as a cause of ovarian cancer, as well as other cancers [299]. Long-term workplace exposure to polycyclic aromatic hydrocarbons (PAH) and already banned chemicals like arylamines is linked to bladder cancer [300, 301]. Chemicals used in the rubber production industry, coal and tin mining and metal processing increase the risk of gastric cancer, and exposure to asbestos and inorganic lead compounds has limited evidence linking them to gastric cancer [302,303,304]. Occupational exposure to trichloroethylene, organochlorine and organophosphate pesticides increases the risk of non-Hodgkin’s lymphoma [305,306,307,308]. Importantly, women’s hairdresser and textile occupations increase non-Hodgkin’s lymphoma risk [308].
Laryngeal cancer risk is elevated with exposure to coal dust, paint fumes, diesel fumes, formaldehyde, nickel, isopropyl alcohol, and asbestos [309,310,311,312,313,314,315,316]. Furthermore, there are other environmental toxicants known to cause cancer that should be considered. Glyphosate, a commonly used herbicide, has raised concerns and is being investigated for its potential carcinogenic effects [317,318,319,320,321]. Benzene, a chemical found in gasoline, industrial solvents, and tobacco smoke, is a known carcinogen associated with various cancers, including leukemia and multiple myeloma [322,323,324,325,326,327,328,329]. Ionizing radiation, such as in-house exposure to radon, is a risk factor for lung cancer and possibly ovarian cancer [330]. Exposure to ultraviolet (UV) radiation is a major risk factor for most melanomas [331].
The cellular and molecular mechanisms of aging that are exacerbated by the aforementioned environmental factors and exposures contribute to tumorigenesis. These mechanisms include increased production of ROS, DNA damage, genetic instability, and various other pathways associated with aging. The interplay between these aging mechanisms and the effects of environmental toxicants contributes to the initiation and progression of malignant diseases. Importantly, there is evidence that the inflammatory milieu maintained by senescent cells contribute to the development of metastases [332,333,334,335,336]. Understanding the impact of environmental toxicants on accelerated aging and cancer development is crucial for implementing preventive measures, promoting environmental regulations, and reducing the burden of cancer in aging populations. Continued research and vigilance are needed to identify and mitigate the risks associated with exposure to environmental toxicants and their role in cancer incidence.
Diseases of the musculoskeletal system
Aging of the musculoskeletal system plays a significant role in the pathogenesis of common diseases that have a negative impact on the quality of life, such as osteoporosis, sarcopenia, rheumatoid arthritis, and osteoarthritis [337]. Osteoporosis, characterized by a dysregulation of osteoclast and osteoblast function, is influenced by age-related mechanisms including the accumulation of senescent cells, heightened inflammation, mitochondrial dysfunction, and dysregulated autophagy [338,339,340,341]. Mechanisms underlying the pathogenesis of osteoporosis involve endocrine changes, dysfunction of myo-satellite cells, increased inflammation, elevated reactive oxygen species production, macromolecular damage, and dysregulation of proteostasis, cellular energetics, and mitochondrial function [342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357]. Sarcopenia, the gradual decline in muscle mass and strength, contributes to frailty and increases the risk of falls and life-threatening bone fractures in older adults, particularly when combined with osteoporosis. The mechanisms underlying sarcopenia involve impaired muscle protein synthesis, dysregulation of anabolic and catabolic signaling pathways, mitochondrial dysfunction, increased oxidative stress, and altered muscle stem cell function, all contributing to the progressive loss of muscle mass and strength with advancing age. Rheumatoid arthritis, a chronic autoimmune disease affecting the joints, also exhibits an increased incidence with age. The aging of the immune system, known as immunosenescence, and subsequent dysregulation of inflammatory processes are implicated in the pathogenesis of rheumatoid arthritis [358].
There are several environmental toxicants that have been linked to the development of musculoskeletal diseases.
Accumulating evidence suggests that both outdoor and indoor air pollution have detrimental effects on musculoskeletal aging. Two comprehensive studies, involving over 9 million individuals aged 65 years and older over an 8-year period, have demonstrated an association between poor air quality and longitudinal bone loss. Individuals living in areas with higher concentrations of PM2.5 particles were found to have a greater risk of osteoporotic fractures [359]. Furthermore, emerging evidence suggests a potential link between ambient air pollution and arthritis [360, 361]. Household air pollution exposure may also play a significant role in the development of arthritis, particularly in low- and middle-income countries [362]. Understanding the effects of air pollution on musculoskeletal aging is of great importance for public health. Mitigating exposure to poor air quality and implementing measures to improve air pollution levels may help prevent or reduce the burden of musculoskeletal diseases associated with aging. Further research is needed to elucidate the underlying mechanisms linking air pollution and musculoskeletal disorders, as well as to explore potential interventions and strategies for promoting healthy musculoskeletal aging in an increasingly polluted environment.
Exposure to lead, commonly found in old paint, contaminated water, and certain occupational settings, has been associated with various musculoskeletal disorders [363,364,365]. Lead exposure can impair bone health, leading to decreased bone mineral density, increased fracture risk, and disturbances in bone remodeling. Certain organophosphate pesticides used in agricultural practices have been implicated in musculoskeletal disorders [366]. Prolonged exposure to these pesticides has been associated with decreased grip strength, muscle weakness, and altered neuromuscular function. Cadmium is a toxic metal present in certain industrial processes, batteries, and cigarette smoke. Prolonged exposure to cadmium has been linked to adverse effects on bone health, including decreased bone mineral density, osteoporosis, and an increased risk of fractures [367]. Polychlorinated biphenyls (PCBs) are persistent organic pollutants that were widely used in electrical equipment and industrial applications. Exposure to PCBs has been associated with adverse effects on the musculoskeletal system [368,369,370].
Certain toxicants have been associated with an increased risk or exacerbation of arthritis. For example, occupational exposure to crystalline silica, commonly found in industries such as mining, construction, and manufacturing, has been linked to an increased risk of developing rheumatoid arthritis [371,372,373,374,375]. It is also possible that prolonged exposure to benzene, a chemical commonly found in industrial settings and certain products such as gasoline, and to vinyl chloride, commonly found in the plastics industry, may also increase the developing of autoimmune diseases, such as arthritis [376, 377].
The mechanisms by which environmental toxicants exacerbate musculoskeletal diseases are complex and involve multiple pathways, including impaired satellite cell function, dysregulation of hormonal signaling, mitochondrial dysfunction, disruption of intracellular signaling pathways involved in muscle protein turnover induction of oxidative stress and inflammation.
Perspectives
In conclusion, the impact of environmental factors on unhealthy cardiovascular, cerebrovascular, and brain aging cannot be overstated. The integration of geroscience, environmental health sciences, and toxicology is crucial in bridging gaps and enhancing our understanding of the relationship between environmental drivers and aging processes. This multidisciplinary approach promotes collaborative efforts and enables a comprehensive assessment of the complex interplay between environmental factors and aging outcomes.
The exposomic framework has emerged as a powerful tool in advancing our understanding of the associations between environmental factors and healthy aging. By capturing the totality of environmental exposures throughout an individual’s life, the exposomic approach provides a holistic perspective on the cumulative effects of these exposures on aging processes. While capturing exposures over a lifetime poses challenges and requires resources, leveraging historical data sources such as retrospective surveys or longitudinal cohorts offers a practical avenue for conducting research in this area. By harnessing existing data, researchers can gain valuable insights into the long-term impact of environmental exposures on aging and identify potential strategies for promoting healthy aging in the population.
Biomarkers of biological age have emerged as valuable tools for assessing the effects of environmental toxicants on aging. These biomarkers provide objective measures of an individual’s physiological state and reflect the cumulative impact of genetic, lifestyle, and environmental factors on the aging process. Telomere length, epigenetic modifications, DNA damage markers, inflammation markers, oxidative stress markers, and mitochondrial function indicators are among the commonly used biomarkers of biological age. Assessing the effects of environmental toxicants on aging through biomarkers allows for a comprehensive understanding of the underlying mechanisms and helps identify individuals at higher risk of accelerated aging or age-related diseases.
Emerging methodologies for determining biological age, such as epigenetic clocks, proteomic clocks, and lipidomic clocks, have brought new insights to the field of aging research [378,379,380,381,382,383]. Epigenetic clocks utilize DNA methylation patterns to predict biological age, while proteomic clocks assess changes in protein levels and modifications associated with aging. By integrating these clocks with physiological measurements, researchers can obtain a more comprehensive understanding of an individual’s biological age and the factors influencing the rate of aging. These methodologies hold great potential for advancing our understanding of healthy aging, identifying individuals at higher risk of age-related diseases, and developing targeted interventions to promote healthier and more vibrant aging.
Integrating the analysis of biological age and biomarkers of aging with epidemiological and toxicological studies allows for a more comprehensive assessment of the relationship between environmental toxicants and aging. This multidimensional approach provides valuable data for developing strategies to mitigate the adverse effects of toxicants, promoting healthier aging, and informing public health policies aimed at reducing exposure to harmful environmental factors. Longitudinal studies tracking changes in biomarker profiles and the subjects’ biological age in response to environmental exposures can provide valuable information on the impact of toxicants over time. Additionally, biomarker assessments can aid in evaluating the effectiveness of interventions or preventive measures aimed at mitigating the detrimental effects of toxicants on aging.
A geroscience approach to environmental health sciences will further identify potential new areas of interdisciplinary research. Environmental toxicants can exacerbate mitochondrial DNA damage and mutagenesis, accelerating mitochondrial aging and promoting dysfunction, cellular energetic dysfunction, apoptosis signaling, and increased production of mitochondria-derived free radicals [384]. Future epidemiological studies should aim to characterize in detail the impacts of environmental chemical exposures on mitochondrial DNA mutagenesis, linking it to the genesis of accelerated aging phenotypes and the incidence of age-related non-communicable diseases.
Furthermore, intersecting area-level indicators with trends in biological aging and the incidence of age-related diseases in a population opens new horizons in epidemiology. By employing this approach, longitudinal studies can lead to a deeper understanding of the role of the exposome in modulating biological aging processes and its contribution to health inequalities in aging. Identifying the role of environmental toxicants in accelerated aging and the increased prevalence of cognitive impairment, dementia, and other age-related diseases in vulnerable populations will advance opportunities for intervention and prevention.
In summary, by advancing our knowledge of the environmental drivers of unhealthy aging and employing an integrative approach, we can develop effective strategies to promote healthier aging, mitigate the detrimental effects of environmental toxicants, and improve the overall well-being of the aging population.
References
WHO Data Platform. https://platform.who.int/data/maternal-newborn-child-adolescent-ageing/indicator-explorer-new/mca/number-of-persons-aged-over-60-years-or-over-(thousands). Accessed 05/01/2023.
National Council on Aging. Chronic inequities: measuring disease cost burden among older adults in the U.S. A health and retirement study analysis. https://ncoa.org/article/get-the-facts-on-healthy-aging. Accessed 05/01/2023.
Chen S, Kuhn M, Prettner K, Bloom DE. The macroeconomic burden of noncommunicable diseases in the United States: estimates and projections. PLoS One. 2018;13:e0206702.
WHO’s work on the UN Decade of Healthy Ageing (2021–2030). https://www.who.int/initiatives/decade-of-healthy-ageing (accessed on 03/15/2023).
World Health Organization. Decade of healthy ageing: baseline report. Summary. Geneva: World Health Organization; 2020.
Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–13.
Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group. GSIG Geroscience. 2017;39:1–5.
Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular aging, a geroscience perspective: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:931–41.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217.
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–32.
Misra BB. The chemical exposome of human aging. Front Genet. 2020;11:574936.
Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14:1847–50.
Dunn AR, O’Connell KMS, Kaczorowski CC. Gene-by-environment interactions in Alzheimer’s disease and Parkinson’s disease. Neurosci Biobehav Rev. 2019;103:73–80.
Finch CE, Kulminski AM. The Alzheimer’s disease exposome. Alzheimers Dement. 2019;15:1123–32.
Huang SY, Yang YX, Chen SD, Li HQ, Zhang XQ, Kuo K, Tan L, Feng L, Dong Q, Zhang C, Yu JT. Investigating causal relationships between exposome and human longevity: a Mendelian randomization analysis. BMC Med. 2021;19:150.
Herceg Z, Ghantous A, Wild CP, Sklias A, Casati L, Duthie SJ, Fry R, Issa JP, Kellermayer R, Koturbash I, Kondo Y, Lepeule J, Lima SCS, Marsit CJ, Rakyan V, Saffery R, Taylor JA, Teschendorff AE, Ushijima T, Vineis P, Walker CL, Waterland RA, Wiemels J, Ambatipudi S, Degli Esposti D, Hernandez-Vargas H. Roadmap for investigating epigenome deregulation and environmental origins of cancer. Int J Cancer. 2018;142:874–82.
Vineis P, Chadeau-Hyam M, Gmuender H, Gulliver J, Herceg Z, Kleinjans J, Kogevinas M, Kyrtopoulos S, Nieuwenhuijsen M, Phillips DH, Probst-Hensch N, Scalbert A, Vermeulen R, Wild CP and Consortium EX. The exposome in practice: design of the EXPOsOMICS project. Int J Hyg Environ Health. 2017;220:142–51.
Agusti A, Melen E, DeMeo DL, Breyer-Kohansal R, Faner R. Pathogenesis of chronic obstructive pulmonary disease: understanding the contributions of gene-environment interactions across the lifespan. Lancet Respir Med. 2022;10:512–24.
Finch CE, Haghani A. Gene-environment interactions and stochastic variations in the gero-exposome. J Gerontol A Biol Sci Med Sci. 2021;76:1740–7.
Kalia V, Belsky DW, Baccarelli AA, Miller GW. An exposomic framework to uncover environmental drivers of aging. Exposome. 2022;2:osac002.
Lan Y, Wu S. Impacts of environmental insults on cardiovascular aging. Curr Environ Health Rep. 2022;9:11–28.
Letellier N, Gutierrez LA, Pilorget C, Artaud F, Descatha A, Ozguler A, Goldberg M, Zins M, Elbaz A, Berr C. Association between occupational exposure to formaldehyde and cognitive impairment. Neurology. 2022;98:e633–40.
Malecki KMC, Andersen JK, Geller AM, Harry GJ, Jackson CL, James KA, Miller GW, Ottinger MA. Integrating environment and aging research: opportunities for synergy and acceleration. Front Aging Neurosci. 2022;14:824921.
Boovarahan SR, Kurian GA. Mitochondrial dysfunction: a key player in the pathogenesis of cardiovascular diseases linked to air pollution. Rev Environ Health. 2018;33:111–22.
Agnihotri A, Aruoma OI. Alzheimer’s disease and Parkinson’s disease: a nutritional toxicology perspective of the impact of oxidative stress, mitochondrial dysfunction, nutrigenomics and environmental chemicals. J Am Coll Nutr. 2020;39:16–27.
Blajszczak C, Bonini MG. Mitochondria targeting by environmental stressors: implications for redox cellular signaling. Toxicology. 2017;391:84–9.
Caito SW, Aschner M. Mitochondrial redox dysfunction and environmental exposures. Antioxid Redox Signal. 2015;23:578–95.
Helley MP, Pinnell J, Sportelli C, Tieu K. Mitochondria: a common target for genetic mutations and environmental toxicants in Parkinson’s disease. Front Genet. 2017;8:177.
McCann MS, Fernandez HR, Flowers SA, Maguire-Zeiss KA. Polychlorinated biphenyls induce oxidative stress and metabolic responses in astrocytes. Neurotoxicology. 2021;86:59–68.
Meyer JN, Leung MC, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS. Mitochondria as a target of environmental toxicants. Toxicol Sci. 2013;134:1–17.
Zhou Z, Goodrich JM, Strakovsky RS. Mitochondrial epigenetics and environmental health: making a case for endocrine disrupting chemicals. Toxicol Sci. 2020;178:16–25.
Brahadeeswaran S, Lateef M, Calivarathan L. An insight into the molecular mechanism of mitochondrial toxicant-induced neuronal apoptosis in Parkinson’s disease. Curr Mol Med. 2023;23:63–75.
Duarte-Hospital C, Tete A, Brial F, Benoit L, Koual M, Tomkiewicz C, Kim MJ, Blanc EB, Coumoul X, Bortoli S. Mitochondrial dysfunction as a hallmark of environmental injury. Cells. 2022;11:110.
Kunovac A, Hathaway QA, Pinti MV, Taylor AD, Hollander JM. Cardiovascular adaptations to particle inhalation exposure: molecular mechanisms of the toxicology. Am J Physiol Heart Circ Physiol. 2020;319:H282–305.
Lopert P, Patel M. Mitochondrial mechanisms of redox cycling agents implicated in Parkinson’s disease. J Neural Transm (Vienna). 2016;123:113–23.
World Health Organization: The Global Health Observatory; Public healthand environment. https://www.who.int/data/gho/data/themes/public-health-and-environment (accessed on 05/29/2023).
Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–67.
Fang X, Crumpler RF, Thomas KN, Mazique JN, Roman RJ, Fan F. Contribution of cerebral microvascular mechanisms to age-related cognitive impairment and dementia. Physiol Int. 2022;109:20–30.
Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res. 2012;110:1109–24.
Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. Geroscience. 2019;41:619–30.
Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci. 2015;70:1355–9.
Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17:e12731.
Tarantini S, Yabluchanskiy A, Csipo T, Fulop G, Kiss T, Balasubramanian P, DelFavero J, Ahire C, Ungvari A, Nyul-Toth A, Farkas E, Benyo Z, Toth A, Csiszar A, Ungvari Z. Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience. 2019;41:533–42.
Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–8.
Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE. Ungvari Z and Csiszar A Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–26.
Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci. 2008;13:5056–70.
Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37-47.
Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–28.
Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–43.
Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37-47.
Wiedenhoeft T, Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Kiss T, Csiszar A, Csiszar A, Ungvari Z. Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience. 2019;41:711–25.
Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–66.
Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, Ungvari A, Toth P, Benyo Z, Sonntag WE, Ungvari Z, Csiszar A. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience. 2021;43:2387–94.
Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyul-Toth A, Yabluchanskiy A, Wren JD, Garman L, Huffman DM, Csiszar A, Ungvari Z. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience. 2020;42:727–48.
Csiszar A, Tarantini S, Fulop GA, Kiss T, Valcarcel-Ares MN, Galvan V, Ungvari Z, Yabluchanskiy A. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience. 2017;39:359–72.
Miller LR, Tarantini S, Nyul-Toth A, Johnston MP, Martin T, Bullen EC, Bickel MA, Sonntag WE, Yabluchanskiy A, Csiszar A, Ungvari ZI, Elliott MH, Conley SM. Increased susceptibility to cerebral microhemorrhages is associated with imaging signs of microvascular degeneration in the retina in an insulin-like growth factor 1 deficient mouse model of accelerated aging. Front Aging Neurosci. 2022;14:788296.
Nyul-Toth A, Tarantini S, Kiss T, Toth P, Galvan V, Tarantini A, Yabluchanskiy A, Csiszar A, Ungvari Z. Increases in hypertension-induced cerebral microhemorrhages exacerbate gait dysfunction in a mouse model of Alzheimer’s disease. Geroscience. 2020;42:1685–98.
Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–79.
Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–20.
Bloom SI, Tucker JR, Lim J, Thomas TG, Stoddard GJ, Lesniewski LA, Donato AJ. Aging results in DNA damage and telomere dysfunction that is greater in endothelial versus vascular smooth muscle cells and is exacerbated in atheroprone regions. Geroscience. 2022;44:2741–55.
Tarantini S, Balasubramanian P, Delfavero J, Csipo T, Yabluchanskiy A, Kiss T, Nyul-Toth A, Mukli P, Toth P, Ahire C, Ungvari A, Benyo Z, Csiszar A, Ungvari Z. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience. 2021;43:2427–40.
Csiszar A, Labinskyy N, Orosz Z, Ungvari Z. Altered mitochondrial energy metabolism may play a role in vascular aging. Med Hypotheses. 2006;67:904–8.
Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173(74–89):e20.
Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyul-Toth A, Yabluchanskiy A, Wren JD, Garman L, Huffman DM, Csiszar A, Ungvari Z. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience. 2020;42:727–48.
Marzetti E, Csiszar A, Dutta D, Balagopal G, Calvani R, Leeuwenburgh C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol. 2013;305:H459–476.
Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–8.
Wenzel P, Schuhmacher S, Kienhofer J, Muller J, Hortmann M, Oelze M, Schulz E, Treiber N, Kawamoto T, Scharffetter-Kochanek K, Munzel T, Burkle A, Bachschmid MM, Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res. 2008;80:280–9.
Zinovkin RA, Romaschenko VP, Galkin II, Zakharova VV, Pletjushkina OY, Chernyak BV, Popova EN. Role of mitochondrial reactive oxygen species in age-related inflammatory activation of endothelium. Aging (Albany NY). 2014;6:661–74.
Csiszar A, Yabluchanskiy A, Ungvari A, Ungvari Z, Tarantini S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience. 2019;41:609–17.
Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–29.
Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292-306.
Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–20.
Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–21.
Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, Gautam T, Zhang XA, Sonntag WE, de Cabo R, Farkas E, Elliott ME, Kinter MT, Deak F, Ungvari Z, Csiszar A. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci. 2018;73:853–63.
Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta E, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–75.
Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363-72.
Ungvari Z, Tarantini S, Nyul-Toth A, Kiss T, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Benyo Z, Csiszar A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: from increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience. 2019;41:727–38.
Ungvari ZI, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson KJ, de Cabo R, Csiszar A. Adaptive induction of NF-E2-Related Factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–40.
Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–9.
Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–9.
Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, DelFavero J, Yabluchanskiy A, Csipo T, Farkas E, Wiley G, Garman L, Csiszar A, Ungvari Z. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience. 2020;42:429–44.
Kiss T, Nyul-Toth A, DelFavero J, Balasubramanian P, Tarantini S, Faakye J, Gulej R, Ahire C, Ungvari A, Yabluchanskiy A, Wiley G, Garman L, Ungvari Z, Csiszar A. Spatial transcriptomic analysis reveals inflammatory foci defined by senescent cells in the white matter, hippocampi and cortical grey matter in the aged mouse brain. Geroscience. 2022;44:661–81.
Tarantini S, Balasubramanian P, Delfavero J, Csipo T, Yabluchanskiy A, Kiss T, Nyul-Toth A, Mukli P, Toth P, Ahire C, Ungvari A, Benyo Z, Csiszar A, Ungvari Z. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience. 2021;43:2427–40.
GBD Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–49.
Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, Daviglus ML, Roux AVD, Gassett AJ, Jacobs DR, Kronmal R, Larson TV, Navas-Acien A, Olives C, Sampson PD, Sheppard L, Siscovick DS, Stein JH, Szpiro AA, Watson KE. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet. 2016;388:696–704.
Hayes RB, Lim C, Zhang Y, Cromar K, Shao Y, Reynolds HR, Silverman DT, Jones RR, Park Y, Jerrett M, Ahn J, Thurston GD. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int J Epidemiol. 2020;49:25–35.
Scheers H, Jacobs L, Casas L, Nemery B, Nawrot TS. Long-term exposure to particulate matter air pollution is a risk factor for stroke. Stroke. 2015;46:3058–66.
Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120:472–95.
Baumgartner J, Clark ML. Studies of household air pollution and subclinical indicators of cardiovascular disease fill important knowledge gaps. J Clin Hypertens (Greenwich). 2016;18:481.
Kanagasabai T, Xie W, Yan L, Zhao L, Carter E, Guo D, Daskalopoulou SS, Chan Q, Elliott P, Ezzati M, Yang X, Xie G, Kelly F, Wu Y, Baumgartner J. Household air pollution and blood pressure, vascular damage, and subclinical indicators of cardiovascular disease in older Chinese adults. Am J Hypertens. 2022;35:121–31.
Noubiap JJ, Essouma M, Bigna JJ. Targeting household air pollution for curbing the cardiovascular disease burden: a health priority in sub-Saharan Africa. J Clin Hypertens (Greenwich). 2015;17:825–9.
Henning RJ, Johnson GT, Coyle JP, Harbison RD. Acrolein can cause cardiovascular disease: a review. Cardiovasc Toxicol. 2017;17:227–36.
Crowley LN, Le BL, Cicalo C, Brown J, Li Y, Kim YJ, Lee JH, Pan JH, Lennon SL, Han BK, Kim JK. Acrolein, An environmental toxicant and its applications to in vivo and in vitro atherosclerosis models: an update. Environ Toxicol Pharmacol. 2022;93:103890.
Klein LW. Pathophysiologic mechanisms of tobacco smoke producing atherosclerosis. Curr Cardiol Rev. 2022;18:e110422203389.
Zirak MR, Mehri S, Karimani A, Zeinali M, Hayes AW, Karimi G. Mechanisms behind the atherothrombotic effects of acrolein, a review. Food Chem Toxicol. 2019;129:38–53.
Messner B, Bernhard D. Smoking and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34:509–15.
Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. 2013;10:219–30.
Churg A, Wang RD, Tai H, Wang X, Xie C, Dai J, Shapiro SD, Wright JL. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am J Respir Crit Care Med. 2003;167:1083–9.
Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol. 2004;24:1031–6.
Kalra VK, Ying Y, Deemer K, Natarajan R, Nadler JL, Coates TD. Mechanism of cigarette smoke condensate induced adhesion of human monocytes to cultured endothelial cells. J Cell Physiol. 1994;160:154–62.
Nagy J, Demaster EG, Wittmann I, Shultz P, Raij L. Induction of endothelial cell injury by cigarette smoke. Endothelium. 1997;5:251–63.
Nordskog BK, Blixt AD, Morgan WT, Fields WR, Hellmann GM. Matrix-degrading and pro-inflammatory changes in human vascular endothelial cells exposed to cigarette smoke condensate. Cardiovasc Toxicol. 2003;3:101–17.
Orosz Z, Csiszar A, Labinskyy N, Smith K, Kaminski PM, Ferdinandy P, Wolin MS, Rivera A, Ungvari Z. Cigarette smoke-induced proinflammatory alterations in the endothelial phenotype: role of NAD(P)H oxidase activation. Am J Physiol Lung Cell Mol Physiol. 2007;292:H130–9.
Ota Y, Kugiyama K, Sugiyama S, Ohgushi M, Matsumura T, Doi H, Ogata N, Oka H, Yasue H. Impairment of endothelium-dependent relaxation of rabbit aortas by cigarette smoke extract–role of free radicals and attenuation by captopril. Atherosclerosis. 1997;131:195–202.
Raij L, DeMaster EG, Jaimes EA. Cigarette smoke-induced endothelium dysfunction: role of superoxide anion. J Hypertens. 2001;19:891–7.
Shen Y, Rattan V, Sultana C, Kalra VK. Cigarette smoke condensate-induced adhesion molecule expression and transendothelial migration of monocytes. Am J Physiol. 1996;270:H1624–33.
Tithof PK, Elgayyar M, Cho Y, Guan W, Fisher AB, Peters-Golden M. Polycyclic aromatic hydrocarbons present in cigarette smoke cause endothelial cell apoptosis by a phospholipase A2-dependent mechanism. Faseb J. 2002;16:1463–4.
Wang H, Ye Y, Zhu M, Cho C. Increased interleukin-8 expression by cigarette smoke extract in endothelial cells. Environ Toxicol Pharmacol. 2000;9:19–23.
Wang J, Wilcken DE, Wang XL. Cigarette smoke activates caspase-3 to induce apoptosis of human umbilical venous endothelial cells. Mol Genet Metab. 2001;72:82–8.
Csiszar A, Podlutsky A, Wolin MS, Losonczy G, Pacher P, Ungvari Z. Oxidative stress and accelerated vascular aging: implications for cigarette smoking. Front Biosci. 2009;14:3128–44.
Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–35.
Anto RJ, Mukhopadhyay A, Shishodia S, Gairola CG, Aggarwal BB. Cigarette smoke condensate activates nuclear transcription factor-kappaB through phosphorylation and degradation of IkappaB(alpha): correlation with induction of cyclooxygenase-2. Carcinogenesis. 2002;23:1511–8.
Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46-57.
Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–76.
Gottdiener JS, Buzkova P, Kahn PA, DeFilippi C, Shah S, Barasch E, Kizer JR, Psaty B, Gardin JM. Relation of cigarette smoking and heart failure in adults ≥65 years of age (from the Cardiovascular Health Study). Am J Cardiol. 2022;168:90–8.
Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep. 2012;14:542–55.
Xu L, Mondal D, Polya DA. Positive association of cardiovascular disease (CVD) with chronic exposure to drinking water arsenic (As) at concentrations below the WHO provisional guideline value: a systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17:2536.
Basha DC, Basha SS, Reddy GR. Lead-induced cardiac and hematological alterations in aging Wistar male rats: alleviating effects of nutrient metal mixture. Biogerontology. 2012;13:359–68.
Bjorklund G, Dadar M, Chirumbolo S, Aaseth J. High content of lead is associated with the softness of drinking water and raised cardiovascular morbidity: a review. Biol Trace Elem Res. 2018;186:384–94.
Moore MR, Meredith PA, Goldberg A, Carr KE, Toner PG, Lawrie TD. Cardiac effects of lead in drinking water of rats. Clin Sci Mol Med. 1975;49:337–41.
Revis NW, Zinsmeister AR, Bull R. Atherosclerosis and hypertension induction by lead and cadmium ions: an effect prevented by calcium ion. Proc Natl Acad Sci U S A. 1981;78:6494–8.
Roncal C, Mu W, Reungjui S, Kim KM, Henderson GN, Ouyang X, Nakagawa T, Johnson RJ. Lead, at low levels, accelerates arteriolopathy and tubulointerstitial injury in chronic kidney disease. Am J Physiol Renal Physiol. 2007;293:F1391–6.
Ryan PB, Huet N, MacIntosh DL. Longitudinal investigation of exposure to arsenic, cadmium, and lead in drinking water. Environ Health Perspect. 2000;108:731–5.
Shen XF, Huang P, Fox DA, Lin Y, Zhao ZH, Wang W, Wang JY, Liu XQ, Chen JY, Luo WJ. Adult lead exposure increases blood-retinal permeability: a risk factor for retinal vascular disease. Neurotoxicology. 2016;57:145–52.
Silva MA, de Oliveira TF, Almenara CC, Broseghini-Filho GB, Vassallo DV, Padilha AS, Silveira EA. Exposure to a low lead concentration impairs contractile machinery in rat cardiac muscle. Biol Trace Elem Res. 2015;167:280–7.
Wu S, Liu H, Zhao H, Wang X, Chen J, Xia D, Xiao C, Cheng J, Zhao Z, He Y. Environmental lead exposure aggravates the progression of Alzheimer’s disease in mice by targeting on blood brain barrier. Toxicol Lett. 2020;319:138–47.
Brake J, Thaxton P, Hester PY. Mercury induced cardiovascular abnormalities in the chicken. Arch Environ Contam Toxicol. 1977;6:269–77.
Wildemann TM, Siciliano SD, Weber LP. The mechanisms associated with the development of hypertension after exposure to lead, mercury species or their mixtures differs with the metal and the mixture ratio. Toxicology. 2016;339:1–8.
Wildemann TM, Weber LP, Siciliano SD. Combined exposure to lead, inorganic mercury and methylmercury shows deviation from additivity for cardiovascular toxicity in rats. J Appl Toxicol. 2015;35:918–26.
Downer MK, Martinez-Gonzalez MA, Gea A, Stampfer M, Warnberg J, Ruiz-Canela M, Salas-Salvado J, Corella D, Ros E, Fito M, Estruch R, Aros F, Fiol M, Lapetra J, Serra-Majem L, Bullo M, Sorli JV, Munoz MA, Garcia-Rodriguez A, Gutierrez-Bedmar M, Gomez-Gracia E, Investigators PS. Mercury exposure and risk of cardiovascular disease: a nested case-control study in the PREDIMED (PREvention with MEDiterranean Diet) study. BMC Cardiovasc Disord. 2017;17:9.
Houston MC. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J Clin Hypertens (Greenwich). 2011;13:621–7.
Hu XF, Lowe M, Chan HM. Mercury exposure, cardiovascular disease, and mortality: a systematic review and dose-response meta-analysis. Environ Res. 2021;193:110538.
Larsen TJ, Jorgensen ME, Larsen CVL, Dahl-Petersen IK, Ronn PF, Bjerregaard P, Byberg S. Whole blood mercury and the risk of cardiovascular disease among the Greenlandic population. Environ Res. 2018;164:310–5.
Mozaffarian D, Shi P, Morris JS, Spiegelman D, Grandjean P, Siscovick DS, Willett WC, Rimm EB. Mercury exposure and risk of cardiovascular disease in two U.S. cohorts. N Engl J Med. 2011;364:1116–25.
Alhamdow A, Lindh C, Albin M, Gustavsson P, Tinnerberg H, Broberg K. Early markers of cardiovascular disease are associated with occupational exposure to polycyclic aromatic hydrocarbons. Sci Rep. 2017;7:9426.
Clark JD 3rd, Serdar B, Lee DJ, Arheart K, Wilkinson JD, Fleming LE. Exposure to polycyclic aromatic hydrocarbons and serum inflammatory markers of cardiovascular disease. Environ Res. 2012;117:132–7.
Rojas GA, Saavedra N, Saavedra K, Hevia M, Morales C, Lanas F, Salazar LA. Polycyclic aromatic hydrocarbons (PAHs) exposure triggers inflammation and endothelial dysfunction in BALB/c mice: a pilot study. Toxics. 2022;10:497.
Chaves RS, Guerreiro CS, Cardoso VV, Benoliel MJ, Santos MM. Hazard and mode of action of disinfection by-products (DBPs) in water for human consumption: evidences and research priorities. Comp Biochem Physiol C Toxicol Pharmacol. 2019;223:53–61.
Kali S, Khan M, Ghaffar MS, Rasheed S, Waseem A, Iqbal MM, Bilal Khan Niazi M, Zafar MI. Occurrence, influencing factors, toxicity, regulations, and abatement approaches for disinfection by-products in chlorinated drinking water: a comprehensive review. Environ Pollut. 2021;281:116950.
Srivastav AL, Patel N, Chaudhary VK. Disinfection by-products in drinking water: occurrence, toxicity and abatement. Environ Pollut. 2020;267:115474.
Chen YC, Chen KF, Andrew Lin KY, Su HP, Wu DN, Lin CH. Evaluation of toxicity of polystyrene microplastics under realistic exposure levels in human vascular endothelial EA.hy926 cells. Chemosphere. 2023;313:137582.
Li Z, Zhu S, Liu Q, Wei J, Jin Y, Wang X, Zhang L. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/beta-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ Pollut. 2020;265:115025.
Persiani E, Cecchettini A, Ceccherini E, Gisone I, Morales MA, Vozzi F. Microplastics: a matter of the heart (and vascular system). Biomedicines. 2023;11:264.
Wang F, Zhang Q, Cui J, Bao B, Deng X, Liu L, Guo MY. Polystyrene microplastics induce endoplasmic reticulum stress, apoptosis and inflammation by disrupting the gut microbiota in carp intestines. Environ Pollut. 2023;323:121233.
Watts GF, Chan DC. Microplastics, cardiometabolic risk, genetics and Alzheimer’s disease. Curr Opin Endocrinol Diabetes Obes. 2022;29:85–6.
Wu D, Feng Y, Wang R, Jiang J, Guan Q, Yang X, Wei H, Xia Y, Luo Y. Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence. J Adv Res. 2023;9:141–50.
Yuan Z, Nag R, Cummins E. Human health concerns regarding microplastics in the aquatic environment - from marine to food systems. Sci Total Environ. 2022;823:153730.
Moulton PV, Yang W. Air pollution, oxidative stress, and Alzheimer’s disease. J Environ Public Health. 2012;2012:472751.
Niemann B, Rohrbach S, Miller MR, Newby DE, Fuster V, Kovacic JC. Oxidative stress and cardiovascular risk: obesity, diabetes, smoking, and pollution: part 3 of a 3-part series. J Am Coll Cardiol. 2017;70:230–51.
Sorensen M, Daneshvar B, Hansen M, Dragsted LO, Hertel O, Knudsen L, Loft S. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect. 2003;111:161–6.
Wilson SJ, Miller MR, Newby DE. Effects of diesel exhaust on cardiovascular function and oxidative stress. Antioxid Redox Signal. 2018;28:819–36.
Daiber A, Munzel T. Special issue “Impact of environmental pollution and stress on redox signaling and oxidative stress pathways.” Redox Biol. 2020;37:101621.
Angeli JK, Cruz Pereira CA, de Oliveira FT, Stefanon I, Padilha AS, Vassallo DV. Cadmium exposure induces vascular injury due to endothelial oxidative stress: the role of local angiotensin II and COX-2. Free Radic Biol Med. 2013;65:838–48.
Daiber A, Kuntic M, Hahad O, Delogu LG, Rohrbach S, Di Lisa F, Schulz R, Munzel T. Effects of air pollution particles (ultrafine and fine particulate matter) on mitochondrial function and oxidative stress - Implications for cardiovascular and neurodegenerative diseases. Arch Biochem Biophys. 2020;696:108662.
Ehsanifar M, Montazeri Z, Taheri MA, Rafati M, Behjati M, Karimian M. Hippocampal inflammation and oxidative stress following exposure to diesel exhaust nanoparticles in male and female mice. Neurochem Int. 2021;145:104989.
Fiorito G, Vlaanderen J, Polidoro S, Gulliver J, Galassi C, Ranzi A, Krogh V, Grioni S, Agnoli C, Sacerdote C, Panico S, Tsai MY, Probst-Hensch N, Hoek G, Herceg Z, Vermeulen R, Ghantous A, Vineis P, Naccarati A, dagger EXc. Oxidative stress and inflammation mediate the effect of air pollution on cardio- and cerebrovascular disease: a prospective study in nonsmokers. Environ Mol Mutagen. 2018;59:234–46.
Gangwar RS, Bevan GH, Palanivel R, Das L, Rajagopalan S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020;34:101545.
Guan L, Geng X, Stone C, Cosky EEP, Ji Y, Du H, Zhang K, Sun Q, Ding Y. PM(2.5) exposure induces systemic inflammation and oxidative stress in an intracranial atherosclerosis rat model. Environ Toxicol. 2019;34:530–8.
Buchner N, Ale-Agha N, Jakob S, Sydlik U, Kunze K, Unfried K, Altschmied J, Haendeler J. Unhealthy diet and ultrafine carbon black particles induce senescence and disease associated phenotypic changes. Exp Gerontol. 2013;48:8–16.
Shiwakoti S, Ko JY, Gong D, Dhakal B, Lee JH, Adhikari R, Gwak Y, Park SH, Jun Choi I, Schini-Kerth VB, Kang KW, Oak MH. Effects of polystyrene nanoplastics on endothelium senescence and its underlying mechanism. Environ Int. 2022;164:107248.
Soundararajan A, Prabu P, Mohan V, Gibert Y, Balasubramanyam M. Novel insights of elevated systemic levels of bisphenol-A (BPA) linked to poor glycemic control, accelerated cellular senescence and insulin resistance in patients with type 2 diabetes. Mol Cell Biochem. 2019;458:171–83.
Yu F, Ye K, Hu Y, Li J, An Y, Qu D. Exposure to polycyclic aromatic hydrocarbons derived from vehicle exhaust gas induces premature senescence in mouse lung fibroblast cells. Mol Med Rep. 2019;19:4326–34.
Dhakal B, Shiwakoti S, Park EY, Kang KW, Schini-Kerth VB, Park SH, Ji HY, Park JS, Ko JY, Oak MH. SGLT2 inhibition ameliorates nano plastics-induced premature endothelial senescence and dysfunction. Sci Rep. 2023;13:6256.
Hodjat M, Rezvanfar MA, Abdollahi M. A systematic review on the role of environmental toxicants in stem cells aging. Food Chem Toxicol. 2015;86:298–308.
Chandy M, Obal D, Wu JC. Elucidating effects of environmental exposure using human-induced pluripotent stem cell disease modeling. EMBO Mol Med. 2022;14:e13260.
Hua T, Kiran S, Li Y, Sang QA. Microplastics exposure affects neural development of human pluripotent stem cell-derived cortical spheroids. J Hazard Mater. 2022;435:128884.
Lai KP, Li JW, Chan TF, Chen A, Lee CYL, Yeung WSB, Wong CKC. Transcriptomic and methylomic analysis reveal the toxicological effect of 2,3,7,8-Tetrachlorodibenzodioxin on human embryonic stem cell. Chemosphere. 2018;206:663–73.
Liu D, Liu NY, Chen LT, Shao Y, Shi XM, Zhu DY. Perfluorooctane sulfonate induced toxicity in embryonic stem cell-derived cardiomyocytes via inhibiting autophagy-lysosome pathway. Toxicol In Vitro. 2020;69:104988.
Liu S, Yang R, Yin N, Wang YL, Faiola F. Environmental and human relevant PFOS and PFOA doses alter human mesenchymal stem cell self-renewal, adipogenesis and osteogenesis. Ecotoxicol Environ Saf. 2019;169:564–72.
Ngalame NNO, Luz AL, Makia N, Tokar EJ. Arsenic alters exosome quantity and cargo to mediate stem cell recruitment into a cancer stem cell-like phenotype. Toxicol Sci. 2018;165:40–9.
Nunes HC, Tavares SC, Garcia HV, Cucielo MS, Dos Santos SAA, Aal MCE, de Golim MA, Justulin LA Jr, Ribeiro AO, Deffune E, Scarano WR, Delella FK. Bisphenol A and 2,3,7,8-tetrachlorodibenzo-p-dioxin at non-cytotoxic doses alter the differentiation potential and cell function of rat adipose-stem cells. Environ Toxicol. 2022;37:2314–23.
Praveena SM, Teh SW, Rajendran RK, Kannan N, Lin CC, Abdullah R, Kumar S. Recent updates on phthalate exposure and human health: a special focus on liver toxicity and stem cell regeneration. Environ Sci Pollut Res Int. 2018;25:11333–42.
Shen J, Wang X, Zhou D, Li T, Tang L, Gong T, Su J, Liang P. Modelling cadmium-induced cardiotoxicity using human pluripotent stem cell-derived cardiomyocytes. J Cell Mol Med. 2018;22:4221–35.
Yang Y, He S, Qi Z, Chai X, Zhao Q, Hu B, Li G, Yu Y. Proliferation toxicity and mechanism of novel mixed bromine/chlorine transformation products of tetrabromobisphenol A on human embryonic stem cell. J Hazard Mater. 2023;449:131050.
Azzouz M, Xu Y, Barregard L, Fagerberg B, Zoller B, Molnar P, Oudin A, Spanne M, Engstrom G, Stockfelt L. Air pollution and biomarkers of cardiovascular disease and inflammation in the Malmo Diet and Cancer cohort. Environ Health. 2022;21:39.
Choi MS, Jeon H, Yoo SM, Lee MS. Activation of the complement system on human endothelial cells by urban particulate matter triggers inflammation-related protein production. Int J Mol Sci. 2021;22:3336.
Daiber A, Kroller-Schon S, Oelze M, Hahad O, Li H, Schulz R, Steven S, Munzel T. Oxidative stress and inflammation contribute to traffic noise-induced vascular and cerebral dysfunction via uncoupling of nitric oxide synthases. Redox Biol. 2020;34:101506.
Hahad O, Lelieveld J, Birklein F, Lieb K, Daiber A, Munzel T. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21:4306.
Han B, Xu J, Zhang Y, Li P, Li K, Zhang N, Han J, Gao S, Wang X, Geng C, Yang W, Zhang L, Bai Z. Associations of exposure to fine particulate matter mass and constituents with systemic inflammation: a cross-sectional study of urban older adults in China. Environ Sci Technol. 2022;56:7244–55.
Hernandez M, Harrington A, Ma Y, Galdanes K, Halzack B, Zhong M, Vaughan J, Sebasco E, Gordon T, Lippmann M, Chen LC. World Trade Center Dust induces airway inflammation while promoting aortic endothelial dysfunction. Toxicol Appl Pharmacol. 2020;400:115041.
Oikonomou E, Lazaros G, Mystakidi VC, Papaioannou N, Theofilis P, Vogiatzi G, Chasikidis C, Fountoulakis P, Papakostantinou MA, Assimakopoulos MN, Barmparesos N, Tasios P, Kaski JC, Tousoulis D. The association of air pollutants exposure with subclinical inflammation and carotid atherosclerosis. Int J Cardiol. 2021;342:108–14.
Orysiak J, Mlynarczyk M, Piec R, Jakubiak A. Lifestyle and environmental factors may induce airway and systemic inflammation in firefighters. Environ Sci Pollut Res Int. 2022;29:73741–68.
Osborne MT, Abohashem S, Naddaf N, Abbasi T, Zureigat H, Mezue K, Ghoneem A, Dar T, Cardeiro AJ, Mehta NN, Rajagopalan S, Fayad ZA, Tawakol A. The combined effect of air and transportation noise pollution on atherosclerotic inflammation and risk of cardiovascular disease events. J Nucl Cardiol. 2023;30:665–79.
Wang T, Chen X, Li H, Chen W, Xu Y, Yao Y, Zhang H, Han Y, Zhang L, Que C, Gong J, Qiu X, Zhu T. Pro-thrombotic changes associated with exposure to ambient ultrafine particles in patients with chronic obstructive pulmonary disease: roles of lipid peroxidation and systemic inflammation. Part Fibre Toxicol. 2022;19:65.
Wu X, Cao X, Lintelmann J, Peters A, Koenig W, Zimmermann R, Schneider A, Wolf K and group KO-S. Assessment of the association of exposure to polycyclic aromatic hydrocarbons, oxidative stress, and inflammation: a cross-sectional study in Augsburg, Germany. Int J Hyg Environ Health. 2022;244:113993.
Xu Z, Wang W, Liu Q, Li Z, Lei L, Ren L, Deng F, Guo X, Wu S. Association between gaseous air pollutants and biomarkers of systemic inflammation: a systematic review and meta-analysis. Environ Pollut. 2022;292:118336.
Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, Hoek G, Hoffmann B, Hoylaerts MF, Kunzli N, Mills N, Pekkanen J, Peters A, Piepoli MF, Rajagopalan S, Storey RF, Esc Working Group on Thrombosis EAfCP, Rehabilitation and Association ESCHF. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36:83–93b.
Akushevich I, Yashkin A, Kovtun M, Kravchenko J, Arbeev K, Yashin AI. Forecasting prevalence and mortality of Alzheimer’s disease using the partitioning models. Exp Gerontol. 2023;174:112133.
Gillis C, Montenigro P, Nejati M, Maserejian N. Estimating prevalence of early Alzheimer’s disease in the United States, accounting for racial and ethnic diversity. Alzheimers Dement. 2023;19:1841–8.
Yang L, Wan W, Yu C, Xuan C, Zheng P, Yan J. Associations between PM(2.5) exposure and Alzheimer’s disease prevalence Among elderly in eastern China. Environ Health. 2022;21:119.
Castillo-Carranza DL, Nilson AN, Van Skike CE, Jahrling JB, Patel K, Garach P, Gerson JE, Sengupta U, Abisambra J, Nelson P, Troncoso J, Ungvari Z, Galvan V, Kayed R. Cerebral microvascular accumulation of Tau oligomers in Alzheimer’s disease and related Tauopathies. Aging Dis. 2017;8:257–66.
Cheung CY, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, Catindig JA, Venketasubramanian N, Yap P, Seow D, Chen CP, Wong TY. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimers Dement. 2014;10:135–42.
Clark LR, Berman SE, Rivera-Rivera LA, Hoscheidt SM, Darst BF, Engelman CD, Rowley HA, Carlsson CM, Asthana S, Turski P, Wieben O, Johnson SC. Macrovascular and microvascular cerebral blood flow in adults at risk for Alzheimer’s disease. Alzheimers Dement (Amst). 2017;7:48–55.
Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611.
Merlini M, Wanner D, Nitsch RM. Tau pathology-dependent remodelling of cerebral arteries precedes Alzheimer’s disease-related microvascular cerebral amyloid angiopathy. Acta Neuropathol. 2016;131:737–52.
Moody DM, Brown WR, Challa VR, Ghazi-Birry HS, Reboussin DM. Cerebral microvascular alterations in aging, leukoaraiosis, and Alzheimer’s disease. Ann N Y Acad Sci. 1997;826:103–16.
Steinman J, Sun HS, Feng ZP. Microvascular alterations in Alzheimer’s disease. Front Cell Neurosci. 2020;14:618986.
Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28:977–86.
Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21:1318–31.
Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–50.
Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78.
Israeli-Korn SD, Masarwa M, Schechtman E, Abuful A, Strugatsky R, Avni S, Farrer LA, Friedland RP, Inzelberg R. Hypertension increases the probability of Alzheimer’s disease and of mild cognitive impairment in an Arab community in northern Israel. Neuroepidemiology. 2010;34:99–105.
Javanshiri K, Waldo ML, Friberg N, Sjovall F, Wickerstrom K, Haglund M, Englund E. Atherosclerosis, hypertension, and diabetes in Alzheimer’s disease, vascular dementia, and mixed dementia: prevalence and presentation. J Alzheimers Dis. 2018;65:1247–58.
Nazarian A, Arbeev KG, Yashkin AP, Kulminski AM. Genetic heterogeneity of Alzheimer’s disease in subjects with and without hypertension. Geroscience. 2019;41:137–54.
Skoog I, Gustafson D. Update on hypertension and Alzheimer’s disease. Neurol Res. 2006;28:605–11.
Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018;14:450–64.
Ionescu-Tucker A, Cotman CW. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol Aging. 2021;107:86–95.
Calderon-Garciduenas L, Herrera-Soto A, Jury N, Maher BA, Gonzalez-Maciel A, Reynoso-Robles R, Ruiz-Rudolph P, van Zundert B, Varela-Nallar L. Reduced repressive epigenetic marks, increased DNA damage and Alzheimer’s disease hallmarks in the brain of humans and mice exposed to particulate urban air pollution. Environ Res. 2020;183:109226.
Pao PC, Patnaik D, Watson LA, Gao F, Pan L, Wang J, Adaikkan C, Penney J, Cam HP, Huang WC, Pantano L, Lee A, Nott A, Phan TX, Gjoneska E, Elmsaouri S, Haggarty SJ, Tsai LH. HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer’s disease. Nat Commun. 2020;11:2484.
Welty S, Thathiah A, Levine AS. DNA damage increases secreted Abeta40 and Abeta42 in neuronal progenitor cells: relevance to Alzheimer’s disease. J Alzheimers Dis. 2022;88:177–90.
Wong GC, Chow KH. DNA damage response-associated cell cycle re-entry and neuronal senescence in brain aging and Alzheimer’s disease. J Alzheimers Dis. 2023;94:S429–51.
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405.
Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017;120:573–91.
Girouard H, Munter LM. The many faces of vascular cognitive impairment. J Neurochem. 2018;144:509–12.
Gorelick PB, Bowler JV. Advances in vascular cognitive impairment. Stroke. 2010;41:e93–8.
Gorelick PB, Counts SE, Nyenhuis D. Vascular cognitive impairment and dementia. Biochim Biophys Acta. 2016;1862:860–8.
Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, Dichgans M. Vascular cognitive impairment and dementia: JACC scientific expert panel. J Am Coll Cardiol. 2019;73:3326–44.
Costanza A, Xekardaki A, Kovari E, Gold G, Bouras C, Giannakopoulos P. Microvascular burden and Alzheimer-type lesions across the age spectrum. J Alzheimers Dis. 2012;32:643–52.
Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kovari E. Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain. 2007;130:2830–6.
van Dinther M, Schram MT, Jansen JFA, Backes WH, Houben A, Berendschot T, Schalkwijk CG, Stehouwer CDA, van Oostenbrugge RJ, Staals J. Extracerebral microvascular dysfunction is related to brain MRI markers of cerebral small vessel disease: The Maastricht Study. Geroscience. 2022;44:147–57.
Nyul-Toth A, Fulop GA, Tarantini S, Kiss T, Ahire C, Faakye JA, Ungvari A, Toth P, Toth A, Csiszar A, Ungvari Z. Cerebral venous congestion exacerbates cerebral microhemorrhages in mice. Geroscience. 2022;44:805–16.
Bagi Z, Kroenke CD, Fopiano KA, Tian Y, Filosa JA, Sherman LS, Larson EB, Keene CD, Degener O’Brien K, Adeniyi PA, Back SA. Association of cerebral microvascular dysfunction and white matter injury in Alzheimer’s disease. Geroscience. 2022;44:1–14.
Szczesniak D, Rymaszewska J, Zimny A, Sasiadek M, Poltyn-Zaradna K, Smith EE, Zatonska K, Zatonski T, Rangarajan S, Yusuf S, Szuba A. Cerebral small vessel disease and other influential factors of cognitive impairment in the middle-aged: a long-term observational cohort PURE-MIND study in Poland. Geroscience. 2021;43:279–95.
Kerkhofs D, Wong SM, Zhang E, Uiterwijk R, Hoff EI, Jansen JFA, Staals J, Backes WH, van Oostenbrugge RJ. Blood-brain barrier leakage at baseline and cognitive decline in cerebral small vessel disease: a 2-year follow-up study. Geroscience. 2021;43:1643–52.
Fan F, Roman RJ. Reversal of cerebral hypoperfusion: a novel therapeutic target for the treatment of AD/ADRD? Geroscience. 2021;43:1065–7.
Toth L, Czigler A, Hegedus E, Komaromy H, Amrein K, Czeiter E, Yabluchanskiy A, Koller A, Orsi G, Perlaki G, Schwarcz A, Buki A, Ungvari Z, Toth PJ. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. Geroscience. 2022;44:2771–83.
Ahire C, Nyul-Toth A, DelFavero J, Gulej R, Faakye JA, Tarantini S, Kiss T, Kuan-Celarier A, Balasubramanian P, Ungvari A, Tarantini A, Nagaraja R, Yan F, Tang Q, Mukli P, Csipo T, Yabluchanskiy A, Campisi J, Ungvari Z, Csiszar A. Accelerated cerebromicrovascular senescence contributes to cognitive decline in a mouse model of paclitaxel (Taxol)-induced chemobrain. Aging Cell. 2023:e13832.
Nyul-Toth A, Tarantini S, DelFavero J, Yan F, Balasubramanian P, Yabluchanskiy A, Ahire C, Kiss T, Csipo T, Lipecz A, Farkas AE, Wilhelm I, Krizbai IA, Tang Q, Csiszar A, Ungvari Z. Demonstration of age-related blood-brain barrier disruption and cerebromicrovascular rarefaction in mice by longitudinal intravital two-photon microscopy and optical coherence tomography. Am J Physiol Heart Circ Physiol. 2021;320:H1370–92.
Weiss B. Vulnerability to pesticide neurotoxicity is a lifetime issue. Neurotoxicology. 2000;21:67–73.
Kukull WA, Larson EB, Bowen JD, McCormick WC, Teri L, Pfanschmidt ML, Thompson JD, O’Meara ES, Brenner DE, van Belle G. Solvent exposure as a risk factor for Alzheimer’s disease: a case-control study. Am J Epidemiol. 2015;141:1059–71 (discussion 1072-9).
Santibanez M, Bolumar F, Garcia AM. Occupational risk factors in Alzheimer’s disease: a review assessing the quality of published epidemiological studies. Occup Environ Med. 2007;64:723–32.
Alemany S, Crous-Bou M, Vilor-Tejedor N, Mila-Aloma M, Suarez-Calvet M, Salvado G, Cirach M, Arenaza-Urquijo EM, Sanchez-Benavides G, Grau-Rivera O, Minguillon C, Fauria K, Kollmorgen G, Domingo Gispert J, Gascon M, Nieuwenhuijsen M, Zetterberg H, Blennow K, Sunyer J, Luis Molinuevo J, study A. Associations between air pollution and biomarkers of Alzheimer’s disease in cognitively unimpaired individuals. Environ Int. 2021;157:106864.
Astrom DO, Adolfsson R, Segersson D, Forsberg B, Oudin A. Local contrasts in concentration of ambient particulate air pollution (PM2.5) and incidence of Alzheimer’s disease and dementia: results from the Betula cohort in Northern Sweden. J Alzheimers Dis. 2021;81:83–5.
Attademo L, Bernardini F. Air pollution as risk factor for mental disorders: in search for a possible link with Alzheimer’s disease and schizophrenia. J Alzheimers Dis. 2020;76:825–30.
Calderon-Garciduenas L, Reynoso-Robles R, Vargas-Martinez J, Gomez-Maqueo-Chew A, Perez-Guille B, Mukherjee PS, Torres-Jardon R, Perry G, Gonzalez-Maciel A. Prefrontal white matter pathology in air pollution exposed Mexico City young urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer’s disease. Environ Res. 2016;146:404–17.
Calderon-Garciduenas L, Torres-Jardon R, Franco-Lira M, Kulesza R, Gonzalez-Maciel A, Reynoso-Robles R, Brito-Aguilar R, Garcia-Arreola B, Revueltas-Ficachi P, Barrera-Velazquez JA, Garcia-Alonso G, Garcia-Rojas E, Mukherjee PS, Delgado-Chavez R. Environmental nanoparticles, SARS-CoV-2 brain involvement, and potential acceleration of Alzheimer’s and Parkinson’s diseases in young urbanites exposed to air pollution. J Alzheimers Dis. 2020;78:479–503.
Fu P, Yung KKL. Air pollution and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2020;77:701–14.
He F, Tang J, Zhang T, Lin J, Li F, Gu X, Chen A, Nevill A, Chen R. Impact of air pollution exposure on the risk of Alzheimer’s disease in China: a community-based cohort study. Environ Res. 2022;205:112318.
Kim SH, Knight EM, Saunders EL, Cuevas AK, Popovech M, Chen LC, Gandy S. Rapid doubling of Alzheimer’s amyloid-beta40 and 42 levels in brains of mice exposed to a nickel nanoparticle model of air pollution. F1000Res. 2012;1:70.
Lin FC, Chen CY, Lin CW, Wu MT, Chen HY, Huang P. Air pollution is associated with cognitive deterioration of Alzheimer’s disease. Gerontology. 2022;68:53–61.
Patten KT, Valenzuela AE, Wallis C, Berg EL, Silverman JL, Bein KJ, Wexler AS, Lein PJ. The effects of chronic exposure to ambient traffic-related air pollution on Alzheimer’s disease phenotypes in wildtype and genetically predisposed male and female rats. Environ Health Perspect. 2021;129:57005.
Power MC. Growing evidence links air pollution exposure to risk of Alzheimer’s disease and related dementia. Brain. 2020;143:8–10.
Shi L, Wu X, Danesh Yazdi M, Braun D, Abu Awad Y, Wei Y, Liu P, Di Q, Wang Y, Schwartz J, Dominici F, Kioumourtzoglou M-A, Zanobetti A. Long-term effects of PM2·5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet Health. 2020;4:e557–65.
Zlokovic BV, Gottesman RF, Bernstein KE, Seshadri S, McKee A, Snyder H, Greenberg SM, Yaffe K, Schaffer CB, Yuan C, Hughes TM, Daemen MJ, Williamson JD, Gonzalez HM, Schneider J, Wellington CL, Katusic ZS, Stoeckel L, Koenig JI, Corriveau RA, Fine L, Galis ZS, Reis J, Wright JD, Chen J. Vascular contributions to cognitive impairment and dementia (VCID): a report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement. 2020;16:1714–33.
Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–34.
Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–8.
Iqubal A, Ahmed M, Ahmad S, Sahoo CR, Iqubal MK, Haque SE. Environmental neurotoxic pollutants: review. Environ Sci Pollut Res Int. 2020;27:41175–98.
McCann MS, Maguire-Zeiss KA. Environmental toxicants in the brain: a review of astrocytic metabolic dysfunction. Environ Toxicol Pharmacol. 2021;84:103608.
Bondy SC. Anthropogenic pollutants may increase the incidence of neurodegenerative disease in an aging population. Toxicology. 2016;341–343:41–6.
Wan C, Liu J, Nie X, Zhao J, Zhou S, Duan Z, Tang C, Liang L, Xu G. 2, 3, 7, 8-Tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PLoS One. 2014;9:e89811.
Fazekas-Pongor V, Fekete M, Balazs P, Arva D, Penzes M, Tarantini S, Urban R, Varga JT. Health-related quality of life of COPD patients aged over 40 years. Physiol Int. 2021;108:261–73.
Fekete M, Fazekas-Pongor V, Balazs P, Tarantini S, Szollosi G, Pako J, Nemeth AN, Varga JT. Effect of malnutrition and body composition on the quality of life of COPD patients. Physiol Int. 2021.
Fekete M, Szollosi G, Tarantini S, Lehoczki A, Nemeth AN, Bodola C, Varga L, Varga JT. Metabolic syndrome in patients with COPD: causes and pathophysiological consequences. Physiol Int. 2022.
Peterfi A, Meszaros A, Szarvas Z, Penzes M, Fekete M, Feher A, Lehoczki A, Csipo T, Fazekas-Pongor V. Comorbidities and increased mortality of COVID-19 among the elderly: a systematic review. Physiol Int. 2022.
Safiri S, Carson-Chahhoud K, Noori M, Nejadghaderi SA, Sullman MJM, Ahmadian Heris J, Ansarin K, Mansournia MA, Collins GS, Kolahi AA, Kaufman JS. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. BMJ. 2022;378:e069679.
MacNee W. Is chronic obstructive pulmonary disease an accelerated aging disease? Ann Am Thorac Soc. 2016;13:S429–37.
Meiners S, Eickelberg O, Konigshoff M. Hallmarks of the ageing lung. Eur Respir J. 2015;45:807–27.
Brandsma CA, de Vries M, Costa R, Woldhuis RR, Konigshoff M, Timens W. Lung ageing and COPD: is there a role for ageing in abnormal tissue repair? Eur Respir Rev. 2017;26.
Mercado N, Ito K, Barnes PJ. Accelerated ageing of the lung in COPD: new concepts. Thorax. 2015;70:482–9.
World Health Organization: ambient (outdoor) air pollution. https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 05/29/2023)
Wen CP, Gao W. PM(2.5): an important cause for chronic obstructive pulmonary disease? Lancet Planet Health. 2018;2:e105–6.
Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung SH, Mortimer K, Perez-Padilla R, Rice MB, Riojas-Rodriguez H, Sood A, Thurston GD, To T, Vanker A, Wuebbles DJ. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air Pollution and Organ Systems. Chest. 2019;155:417–26.
GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8:585–96.
Urban VS, Cegledi A, Mikala G. Multiple myeloma, a quintessential malignant disease of aging: a geroscience perspective on pathogenesis and treatment. Geroscience. 2023;45:727–46.
Schmidlin K, Spoerri A, Egger M, Zwahlen M, Stuck A, Clough-Gorr KM, Swiss NC. Cancer, a disease of aging (part 1) - trends in older adult cancer mortality in Switzerland 1991–2008. Swiss Med Wkly. 2012;142:w13637.
Schmidlin K, Spoerri A, Egger M, Zwahlen M, Stuck A, Clough-Gorr KM, Swiss NC. Cancer, a disease of aging (part 2) - risk factors for older adult cancer mortality in Switzerland 1991–2008. Swiss Med Wkly. 2012;142:w13607.
Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4.
Garcia AM, Busuttil RA, Calder RB, Dolle ME, Diaz V, McMahan CA, Bartke A, Nelson J, Reddick R, Vijg J. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech Ageing Dev. 2008;129:528–33.
Heydari AR, Unnikrishnan A, Lucente LV, Richardson A. Caloric restriction and genomic stability. Nucleic Acids Res. 2007;35:7485–96.
Kritchevsky D. Caloric restriction and experimental mammary carcinogenesis. Breast Cancer Res Treat. 1997;46:161–7.
Michels KB, Ekbom A. Caloric restriction and incidence of breast cancer. JAMA. 2004;291:1226–30.
Weindruch R. Effect of caloric restriction on age-associated cancers. Exp Gerontol. 1992;27:575–81.
Anisimov VN. Mutant and genetically modified mice as models for studying the relationship between aging and carcinogenesis. Mech Ageing Dev. 2001;122:1221–55.
Sun LY, Fang Y, Patki A, Koopman JJ, Allison DB, Hill CM, Masternak MM, Darcy J, Wang J, McFadden S, Bartke A. Longevity is impacted by growth hormone action during early postnatal period. Elife. 2017;6.
Morales-Valencia J, David G. The contribution of physiological and accelerated aging to cancer progression through senescence-induced inflammation. Front Oncol. 2021;11:747822.
Takeda T, Matsushita T, Kurozumi M, Takemura K, Higuchi K, Hosokawa M. Pathobiology of the senescence-accelerated mouse (SAM). Exp Gerontol. 1997;32:117–27.
Zhang Y, Unnikrishnan A, Deepa SS, Liu Y, Li Y, Ikeno Y, Sosnowska D, Van Remmen H, Richardson A. A new role for oxidative stress in aging: the accelerated aging phenotype in Sod1(-/)(-) mice is correlated to increased cellular senescence. Redox Biol. 2017;11:30–7.
Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, Hoffmann B, Fischer P, Nieuwenhuijsen MJ, Brunekreef B, Xun WW, Katsouyanni K, Dimakopoulou K, Sommar J, Forsberg B, Modig L, Oudin A, Oftedal B, Schwarze PE, Nafstad P, De Faire U, Pedersen NL, Ostenson CG, Fratiglioni L, Penell J, Korek M, Pershagen G, Eriksen KT, Sorensen M, Tjonneland A, Ellermann T, Eeftens M, Peeters PH, Meliefste K, Wang M, Bueno-de-Mesquita B, Key TJ, de Hoogh K, Concin H, Nagel G, Vilier A, Grioni S, Krogh V, Tsai MY, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Tamayo I, Amiano P, Dorronsoro M, Trichopoulou A, Bamia C, Vineis P, Hoek G. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013;14:813–22.
Straif K, Cohen A, Samet J. Air pollution and cancer. IV. series. Geneva: International Agency for Research on Cancer; 2013.
Tagliabue G, Borgini A, Tittarelli A, van Donkelaar A, Martin RV, Bertoldi M, Fabiano S, Maghini A, Codazzi T, Scaburri A, Favia I, Cau A, Barigelletti G, Tessandori R, Contiero P. Atmospheric fine particulate matter and breast cancer mortality: a population-based cohort study. BMJ Open. 2016;6:e012580.
Barta JA, Powell CA and Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019;85.
Cheng ES, Egger S, Hughes S, Weber M, Steinberg J, Rahman B, Worth H, Ruano-Ravina A, Rawstorne P, Yu XQ. Systematic review and meta-analysis of residential radon and lung cancer in never-smokers. Eur Respir Rev. 2021;30.
Ciabattini M, Rizzello E, Lucaroni F, Palombi L, Boffetta P. Systematic review and meta-analysis of recent high-quality studies on exposure to particulate matter and risk of lung cancer. Environ Res. 2021;196:110440.
Corrales L, Rosell R, Cardona AF, Martin C, Zatarain-Barron ZL, Arrieta O. Lung cancer in never smokers: the role of different risk factors other than tobacco smoking. Crit Rev Oncol Hematol. 2020;148:102895.
Honaryar MK, Lunn RM, Luce D, Ahrens W, Mannetje TA, Hansen J, Bouaoun L, Loomis D, Byrnes G, Vilahur N, Stayner L, Guha N. Welding fumes and lung cancer: a meta-analysis of case-control and cohort studies. Occup Environ Med. 2019;76:422–31.
Li J, Ran J, Chen LC, Costa M, Huang Y, Chen X, Tian L. Bituminous coal combustion and Xuan Wei Lung cancer: a review of the epidemiology, intervention, carcinogens, and carcinogenesis. Arch Toxicol. 2019;93:573–83.
Rodriguez-Martinez A, Torres-Duran M, Barros-Dios JM, Ruano-Ravina A. Residential radon and small cell lung cancer. A Syst Rev Cancer Lett. 2018;426:57–62.
Shankar A, Dubey A, Saini D, Singh M, Prasad CP, Roy S, Bharati SJ, Rinki M, Singh N, Seth T, Khanna M, Sethi N, Kumar S, Sirohi B, Mohan A, Guleria R, Rath GK. Environmental and occupational determinants of lung cancer. Transl Lung Cancer Res. 2019;8:S31–49.
Soza-Ried C, Bustamante E, Caglevic C, Rolfo C, Sirera R, Marsiglia H. Oncogenic role of arsenic exposure in lung cancer: a forgotten risk factor. Crit Rev Oncol Hematol. 2019;139:128–33.
Stepanek L, Sevcikova J, Horakova D, Patel MS, Durdakova R. Public health burden of secondhand smoking: case reports of lung cancer and a literature review. Int J Environ Res Public Health. 2022;19.
Wang N, Mengersen K, Kimlin M, Zhou M, Tong S, Fang L, Wang B, Hu W. Lung cancer and particulate pollution: a critical review of spatial and temporal analysis evidence. Environ Res. 2018;164:585–96.
Du CL, Wang JD. Increased morbidity odds ratio of primary liver cancer and cirrhosis of the liver among vinyl chloride monomer workers. Occup Environ Med. 1998;55:528–32.
Lewis R, Rempala G, Dell LD, Mundt KA. Vinyl chloride and liver and brain cancer at a polymer production plant in Louisville. Kentucky J Occup Environ Med. 2003;45:533–7.
Mundt KA, Dell LD, Crawford L, Gallagher AE. Quantitative estimated exposure to vinyl chloride and risk of angiosarcoma of the liver and hepatocellular cancer in the US industry-wide vinyl chloride cohort: mortality update through 2013. Occup Environ Med. 2017;74:709–16.
Towle KM, Benson SM, Egnot NS, Marsh GM. An ecological evaluation of vinyl chloride exposure and liver cancer incidence and mortality in Texas. J Clin Transl Hepatol. 2021;9:99–105.
Wang W, Cheng S, Zhang D. Association of inorganic arsenic exposure with liver cancer mortality: a meta-analysis. Environ Res. 2014;135:120–5.
Alexander DD, Kelsh MA, Mink PJ, Mandel JH, Basu R, Weingart M. A meta-analysis of occupational trichloroethylene exposure and liver cancer. Int Arch Occup Environ Health. 2007;81:127–43.
Vlaanderen J, Straif K, Pukkala E, Kauppinen T, Kyyronen P, Martinsen JI, Kjaerheim K, Tryggvadottir L, Hansen J, Sparen P, Weiderpass E. Occupational exposure to trichloroethylene and perchloroethylene and the risk of lymphoma, liver, and kidney cancer in four Nordic countries. Occup Environ Med. 2013;70:393–401.
Camargo MC, Stayner LT, Straif K, Reina M, Al-Alem U, Demers PA, Landrigan PJ. Occupational exposure to asbestos and ovarian cancer: a meta-analysis. Environ Health Perspect. 2011;119:1211–7.
Burstyn I, Kromhout H, Johansen C, Langard S, Kauppinen T, Shaham J, Ferro G, Boffetta P. Bladder cancer incidence and exposure to polycyclic aromatic hydrocarbons among asphalt pavers. Occup Environ Med. 2007;64:520–6.
Clavel J, Mandereau L, Limasset JC, Hemon D, Cordier S. Occupational exposure to polycyclic aromatic hydrocarbons and the risk of bladder cancer: a French case-control study. Int J Epidemiol. 1994;23:1145–53.
Raj A, Mayberry JF, Podas T. Occupation and gastric cancer. Postgrad Med J. 2003;79:252–8.
Eguchi H, Wada K, Prieto-Merino D, Smith DR. Lung, gastric and colorectal cancer mortality by occupation and industry among working-aged men in Japan. Sci Rep. 2017;7:43204.
Yoshinaga Y, Tanaka H, Wada K, Ikeda S. Gastric cancer mortality rates by occupation and industry among male and female workers aged 25–64 years in Japan. Ind Health. 2020;58:554–64.
Boffetta P, de Vocht F. Occupation and the risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:369–72.
Cano MI, Pollan M. Non-Hodgkin’s lymphomas and occupation in Sweden. Int Arch Occup Environ Health. 2001;74:443–9.
Schenk M, Purdue MP, Colt JS, Hartge P, Blair A, Stewart P, Cerhan JR, De Roos AJ, Cozen W, Severson RK. Occupation/industry and risk of non-Hodgkin’s lymphoma in the United States. Occup Environ Med. 2009;66:23–31.
Mannetje TA, De Roos AJ, Boffetta P, Vermeulen R, Benke G, Fritschi L, Brennan P, Foretova L, Maynadie M, Becker N, Nieters A, Staines A, Campagna M, Chiu B, Clavel J, de Sanjose S, Hartge P, Holly EA, Bracci P, Linet MS, Monnereau A, Orsi L, Purdue MP, Rothman N, Lan Q, Kane E, Costantini AS, Miligi L, Spinelli JJ, Zheng T, Cocco P, Kricker A. Occupation and risk of non-Hodgkin lymphoma and its subtypes: a pooled analysis from the InterLymph Consortium. Environ Health Perspect. 2016;124:396–405.
Baan R, Grosse Y, Straif K, Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, Group WHOIAfRoCMW. A review of human carcinogens–Part F: chemical agents and related occupations. Lancet Oncol. 2009;10:1143–4.
Barul C, Carton M, Radoi L, Menvielle G, Pilorget C, Bara S, Stucker I, Luce D, group Is. Occupational exposure to petroleum-based and oxygenated solvents and hypopharyngeal and laryngeal cancer in France: the ICARE study. BMC Cancer. 2018;18:388.
Becher H, Ramroth H, Ahrens W, Risch A, Schmezer P, Dietz A. Occupation, exposure to polycyclic aromatic hydrocarbons and laryngeal cancer risk. Int J Cancer. 2005;116:451–7.
Dietz A, Ramroth H, Urban T, Ahrens W, Becher H. Exposure to cement dust, related occupational groups and laryngeal cancer risk: results of a population based case-control study. Int J Cancer. 2004;108:907–11.
Hall AL, Kromhout H, Schuz J, Peters S, Portengen L, Vermeulen R, Agudo A, Ahrens W, Boffetta P, Brennan P, Canova C, Conway DI, Curado MP, Daudt AW, Fernandez L, Hashibe M, Healy CM, Holcatova I, Kjaerheim K, Koifman R, Lagiou P, Luce D, Macfarlane GJ, Menezes A, Menvielle G, Polesel J, Ramroth H, Richiardi L, Stucker I, Thomson P, Vilensky M, Wunsch-Filho V, Yuan-Chin AL, Znaor A, Straif K, Olsson A. Laryngeal cancer risks in workers exposed to lung carcinogens: exposure-effect analyses using a quantitative job exposure matrix. Epidemiology. 2020;31:145–54.
Peng WJ, Mi J, Jiang YH. Asbestos exposure and laryngeal cancer mortality. Laryngoscope. 2016;126:1169–74.
Ramroth H, Dietz A, Ahrens W, Becher H. Occupational wood dust exposure and the risk of laryngeal cancer: a population based case-control study in Germany. Am J Ind Med. 2008;51:648–55.
Shangina O, Brennan P, Szeszenia-Dabrowska N, Mates D, Fabianova E, Fletcher T, Tmannetje A, Boffetta P, Zaridze D. Occupational exposure and laryngeal and hypopharyngeal cancer risk in central and eastern Europe. Am J Epidemiol. 2006;164:367–75.
Andreotti G, Koutros S, Hofmann JN, Sandler DP, Lubin JH, Lynch CF, Lerro CC, De Roos AJ, Parks CG, Alavanja MC, Silverman DT, Beane Freeman LE. Glyphosate use and cancer incidence in the agricultural health study. J Natl Cancer Inst. 2018;110:509–16.
Davoren MJ, Schiestl RH. Glyphosate-based herbicides and cancer risk: a post-IARC decision review of potential mechanisms, policy and avenues of research. Carcinogenesis. 2018;39:1207–15.
Franke AA, Li X, Shvetsov YB, Lai JF. Pilot study on the urinary excretion of the glyphosate metabolite aminomethylphosphonic acid and breast cancer risk: the Multiethnic Cohort study. Environ Pollut. 2021;277:116848.
Marino M, Mele E, Viggiano A, Nori SL, Meccariello R, Santoro A. Pleiotropic outcomes of glyphosate exposure: from organ damage to effects on inflammation, cancer, reproduction and development. Int J Mol Sci. 2021;22:12606.
Ward EM. Glyphosate use and cancer incidence in the agricultural health study: an epidemiologic perspective. J Natl Cancer Inst. 2018;110:446–7.
Chaiklieng S, Suggaravetsiri P, Autrup H. Risk assessment on benzene exposure among gasoline station workers. Int J Environ Res Public Health. 2019;16:2545.
Costantini AS, Benvenuti A, Vineis P, Kriebel D, Tumino R, Ramazzotti V, Rodella S, Stagnaro E, Crosignani P, Amadori D, Mirabelli D, Sommani L, Belletti I, Troschel L, Romeo L, Miceli G, Tozzi GA, Mendico I, Maltoni SA, Miligi L. Risk of leukemia and multiple myeloma associated with exposure to benzene and other organic solvents: evidence from the Italian Multicenter Case-control study. Am J Ind Med. 2008;51:803–11.
Infante PF. Benzene exposure and multiple myeloma: a detailed meta-analysis of benzene cohort studies. Ann N Y Acad Sci. 2006;1076:90–109.
Kawasaki S, Takizawa H, Takami K, Desaki M, Okazaki H, Kasama T, Kobayashi K, Yamamoto K, Nakahara K, Tanaka M, Sagai M, Ohtoshi T. Benzene-extracted components are important for the major activity of diesel exhaust particles: effect on interleukin-8 gene expression in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2001;24:419–26.
Maltoni C, Ciliberti A, Cotti G, Conti B, Belpoggi F. Benzene, an experimental multipotential carcinogen: results of the long-term bioassays performed at the Bologna Institute of Oncology. Environ Health Perspect. 1989;82:109–24.
Savitz DA, Andrews KW. Risk of myelogenous leukaemia and multiple myeloma in workers exposed to benzene. Occup Environ Med. 1996;53:357–8.
Sonoda T, Nagata Y, Mori M, Ishida T, Imai K. Meta-analysis of multiple myeloma and benzene exposure. J Epidemiol. 2001;11:249–54.
Teitelbaum DT, Brautbar N. Benzene and multiple myeloma: appraisal of the scientific evidence. Blood. 2000;95:2995–7.
Gaskin J, Coyle D, Whyte J, Krewksi D. Global estimate of lung cancer mortality attributable to residential radon. Environ Health Perspect. 2018;126:057009.
Rivers JK. Is there more than one road to melanoma? The Lancet. 2004;363:728–30.
Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705.
Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40.
Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AM, Alston S, Academia EC, Kilmarx S, Valdovinos A, Wang B, de Bruin A, Kennedy BK, Melov S, Zhou D, Sharpless NE, Muss H, Campisi J. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7:165–76.
Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–7.
Lecot P, Alimirah F, Desprez PY, Campisi J, Wiley C. Context-dependent effects of cellular senescence in cancer development. Br J Cancer. 2016;114:1180–4.
Vincent HK, Raiser SN, Vincent KR. The aging musculoskeletal system and obesity-related considerations with exercise. Ageing Res Rev. 2012;11:361–73.
Guo Y, Yang TL, Liu YZ, Shen H, Lei SF, Yu N, Chen J, Xu T, Cheng Y, Tian Q, Yu P, Deng HW. Mitochondria-wide association study of common variants in osteoporosis. Ann Hum Genet. 2011;75:569–74.
Foger-Samwald U, Kerschan-Schindl K, Butylina M, Pietschmann P. Age related osteoporosis: targeting cellular senescence. Int J Mol Sci. 2022;23:2701.
Guo Y, Jia X, Cui Y, Song Y, Wang S, Geng Y, Li R, Gao W, Fu D. Sirt3-mediated mitophagy regulates AGEs-induced BMSCs senescence and senile osteoporosis. Redox Biol. 2021;41:101915.
Pignolo RJ, Law SF, Chandra A. Bone aging, cellular senescence, and osteoporosis. JBMR Plus. 2021;5:e10488.
Coen PM, Musci RV, Hinkley JM, Miller BF. Mitochondria as a target for mitigating sarcopenia. Front Physiol. 2018;9:1883.
Daussin FN, Boulanger E, Lancel S. From mitochondria to sarcopenia: role of inflammaging and RAGE-ligand axis implication. Exp Gerontol. 2021;146:111247.
Del Campo A, Contreras-Hernandez I, Castro-Sepulveda M, Campos CA, Figueroa R, Tevy MF, Eisner V, Casas M, Jaimovich E. Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice. Aging (Albany NY). 2018;10:34–55.
Sataranatarajan K, Pharaoh G, Brown JL, Ranjit R, Piekarz KM, Street K, Wren JD, Georgescu C, Kinter C, Kinter M, Freeman WM, Richardson A, Van Remmen H. Molecular changes in transcription and metabolic pathways underlying muscle atrophy in the CuZnSOD null mouse model of sarcopenia. Geroscience. 2020;42:1101–18.
Lagerwaard B, Nieuwenhuizen AG, de Boer VCJ, Keijer J. In vivo assessment of mitochondrial capacity using NIRS in locomotor muscles of young and elderly males with similar physical activity levels. Geroscience. 2020;42:299–310.
Siegel MP, Kruse SE, Percival JM, Goh J, White CC, Hopkins HC, Kavanagh TJ, Szeto HH, Rabinovitch PS, Marcinek DJ. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell. 2013;12:763–71.
Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–6.
Romanick MA, Rakoczy SG, Brown-Borg HM. Long-lived Ames dwarf mouse exhibits increased antioxidant defense in skeletal muscle. Mech Ageing Dev. 2004;125:269–81.
Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–11.
Schwarze SR, Lee CM, Chung SS, Roecker EB, Weindruch R, Aiken JM. High levels of mitochondrial DNA deletions in skeletal muscle of old rhesus monkeys. Mech Ageing Dev. 1995;83:91–101.
Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1:637–9.
Ubaida-Mohien C, Lyashkov A, Gonzalez-Freire M, Tharakan R, Shardell M, Moaddel R, Semba RD, Chia CW, Gorospe M, Sen R, Ferrucci L. Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. Elife. 2019;8:e49874.
Yeo D, Kang C, Ji LL. Aging alters acetylation status in skeletal and cardiac muscles. Geroscience. 2020;42:963–76.
Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–43.
Zhang Y, Ikeno Y, Qi W, Chaudhuri A, Li Y, Bokov A, Thorpe SR, Baynes JW, Epstein C, Richardson A, Van Remmen H. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci. 2009;64:1212–20.
Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, Grune T. Sarcopenia - molecular mechanisms and open questions. Ageing Res Rev. 2021;65:101200.
Chalan P, van den Berg A, Kroesen BJ, Brouwer L, Boots A. Rheumatoid arthritis, immunosenescence and the hallmarks of aging. Curr Aging Sci. 2015;8:131–46.
Prada D, Zhong J, Colicino E, Zanobetti A, Schwartz J, Dagincourt N, Fang SC, Kloog I, Zmuda JM, Holick M, Herrera LA, Hou L, Dominici F, Bartali B, Baccarelli AA. Association of air particulate pollution with bone loss over time and bone fracture risk: analysis of data from two independent studies. Lancet Planet Health. 2017;1:e337–47.
Berenbaum F, Wallace IJ, Lieberman DE, Felson DT. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2018;14:674–81.
Shin J, Lee J, Lee J, Ha E-H. Association between exposure to ambient air pollution and rheumatoid arthritis in adults. International Journal of Environmental Research and Public Health. 2019;16.
Yamamoto SS, Yacyshyn E, Jhangri GS, Chopra A, Parmar D, Jones CA. Household air pollution and arthritis in low-and middle-income countries: cross-sectional evidence from the World Health Organization’s study on Global Ageing and Adult Health. PLoS One. 2019;14:e0226738.
Ravibabu K, Bagepally BS, Barman T. Association of musculoskeletal disorders and inflammation markers in workers exposed to lead (Pb) from Pb-battery manufacturing plant. Indian J Occup Environ Med. 2019;23:68–72.
Manocha A, Srivastava LM, Bhargava S. Lead as a risk factor for osteoporosis in post-menopausal women. Indian J Clin Biochem. 2017;32:261–5.
Sun Y, Sun D, Zhou Z, Zhu G, Zhang H, Chang X, Lei L, Jin T. Osteoporosis in a Chinese population due to occupational exposure to lead. Am J Ind Med. 2008;51:436–42.
Kirkhorn SR, Schenker MB. Current health effects of agricultural work: respiratory disease, cancer, reproductive effects, musculoskeletal injuries, and pesticide-related illnesses. J Agric Saf Health. 2002;8:199–214.
Ciosek Z, Kot K, Rotter I. Iron, zinc, copper, cadmium, mercury, and bone tissue. Int J Environ Res Public Health. 2023;20:2197.
Anderson HA, Lilis R, Selikoff IJ, Rosenman KD, Valciukas JA, Freedman S. Unanticipated prevalence of symptoms among dairy farmers in Michigan and Wisconsin. Environ Health Perspect. 1978;23:217–26.
Broding HC, Schettgen T, Hillert A, Angerer J, Goen T, Drexler H. Subjective complaints in persons under chronic low-dose exposure to lower polychlorinated biphenyls (PCBs). Int J Hyg Environ Health. 2008;211:648–57.
Guo YL, Lin CJ, Yao WJ, Ryan JJ, Hsu CC. Musculoskeletal changes in children prenatally exposed to polychlorinated biphenyls and related compounds (Yu-Cheng children). J Toxicol Environ Health. 1994;41:83–93.
Boudigaard SH, Schlunssen V, Vestergaard JM, Sondergaard K, Toren K, Peters S, Kromhout H, Kolstad HA. Occupational exposure to respirable crystalline silica and risk of autoimmune rheumatic diseases: a nationwide cohort study. Int J Epidemiol. 2021;50:1213–26.
Cavalin C, Lescoat A, Sigaux J, Macchi O, Ballerie A, Catinon M, Vincent M, Semerano L, Boissier MC, Rosental PA. Crystalline silica exposure in patients with rheumatoid arthritis and systemic sclerosis: a nationwide cross-sectional survey. Rheumatology (Oxford). 2023;62:2707–15.
Min YS, Kim MG, Ahn YS. Rheumatoid arthritis in silica-exposed workers. Int J Environ Res Public Health. 2021;18:12776.
Morotti A, Sollaku I, Franceschini F, Cavazzana I, Fredi M, Sala E, De Palma G. Systematic review and meta-analysis on the association of occupational exposure to free crystalline silica and rheumatoid arthritis. Clin Rev Allergy Immunol. 2022;62:333–45.
Wrangel O, Graff P, Bryngelsson IL, Fornander L, Wiebert P, Vihlborg P. Silica dust exposure increases risk for rheumatoid arthritis: a Swedish National Registry Case-Control Study. J Occup Environ Med. 2021;63:951–5.
Veraldi A, Costantini AS, Bolejack V, Miligi L, Vineis P, van Loveren H. Immunotoxic effects of chemicals: a matrix for occupational and environmental epidemiological studies. Am J Ind Med. 2006;49:1046–55.
Biton J, Saidenberg-Kermanac’h N, Decker P, Boissier MC, Semerano L, Sigaux J. The exposome in rheumatoid arthritis. Joint Bone Spine. 2022;89:105455.
Jokai M, Torma F, McGreevy KM, Koltai E, Bori Z, Babszki G, Bakonyi P, Gombos Z, Gyorgy B, Aczel D, Toth L, Osvath P, Fridvalszky M, Teglas T, Posa A, Kujach S, Olek R, Kawamura T, Seki Y, Suzuki K, Tanisawa K, Goto S, Kerepesi C, Boldogh I, Ba X, Davies KJA, Horvath S, Radak Z. DNA methylation clock DNAmFitAge shows regular exercise is associated with slower aging and systemic adaptation. Geroscience. 2023. https://doi.org/10.1007/s11357-023-00826-1.
Oblak L, van der Zaag J, Higgins-Chen AT, Levine ME, Boks MP. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res Rev. 2021;69:101348.
Grodstein F, Lemos B, Yu L, Klein HU, Iatrou A, Buchman AS, Shireby GL, Mill J, Schneider JA, De Jager PL, Bennett DA. The association of epigenetic clocks in brain tissue with brain pathologies and common aging phenotypes. Neurobiol Dis. 2021;157:105428.
Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–84.
Marioni RE, Shah S, McRae AF, Ritchie SJ, Muniz-Terrera G, Harris SE, Gibson J, Redmond P, Cox SR, Pattie A, Corley J, Taylor A, Murphy L, Starr JM, Horvath S, Visscher PM, Wray NR, Deary IJ. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol. 2015;44:1388–96.
Subedi P, Palma-Gudiel H, Fiehn O, Best LG, Lee ET, Howard BV, Zhao J. Lipidomics profiling of biological aging in American Indians: the Strong Heart Family Study. Geroscience. 2023;45:359–69.
Leuthner TC, Meyer JN. Mitochondrial DNA mutagenesis: feature of and biomarker for environmental exposures and aging. Curr Environ Health Rep. 2021;8:294–308.
Görgey A. Über die festen, flüchtigen, fetten Säueren des Cocusnussöles Sitzungsberichte der mathematisch-naturwissenschaftlichen Classe der k Akademie der Wissenschaften in Wien Vienna: Akademie der Wissenschaften in Wien.;1848(3): 208—227.
Funding
Open access funding provided by Semmelweis University. Drs. Anna Csiszar and Zoltan Ungvari were supported by the National Institute on Aging (RF1AG072295, R01AG055395, R01AG068295; R01AG070915, R03AG070479), the National Institute of Neurological Disorders and Stroke (R01NS100782), the National Cancer Institute (R01CA255840), the Presbyterian Health Foundation, the Reynolds Foundation, the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (P20GM125528). Project no. TKP2021-NKTA-47 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme. Funding for the project through the National Cardiovascular Laboratory Program (RRF-2.3.1–21-2022–00003) was provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund. Project no. 135784 has also been implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the K_20 funding scheme. This work was also supported by grants from the European University for Well-Being (EUniWell) program (grant agreement number: 101004093/ EUniWell/EAC-A02-2019 / EAC-A02-2019–1). Prof. Dr. Zoltan Benyo was supported by grants from the Ministry of Innovation and Technology of Hungary from the NRDI Fund (2020–1.1.6-JÖVŐ-2021–00010, RRF-2.1.2–21-2022–00010, and TKP2021-EGA-25). The authors acknowledge the scientific contributions of Gen. Artúr Görgei [385]. The views expressed are those of the author and not necessarily those of the NIH or of the other funding agencies. The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The 3.5 version of ChatGPT, developed by OpenAI, proved to be a useful tool in refining the writing and enhancing the clarity of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr. Stefano Tarantini, Dr. Andriy Yabluchanskiy, Dr. Shannon Conley, Dr. Anna Csiszar, Dr. Zoltan Benyo and Dr. Roza Adany serve as Associate Editors for GeroScience. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Pandics, T., Major, D., Fazekas-Pongor, V. et al. Exposome and unhealthy aging: environmental drivers from air pollution to occupational exposures. GeroScience 45, 3381–3408 (2023). https://doi.org/10.1007/s11357-023-00913-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00913-3