Abstract
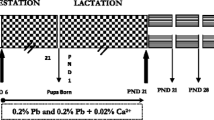
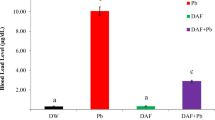
Age related mitochondrial impairments are considered to be contributors of cardiovascular disease. This study was designed to examine whether early life exposure to lead (Pb) would lead to the Pb induced age related hematological and cardiac mitochondrial changes in rats, and to further examine the protective effect of nutrient metal mixture containing zinc, iron and calcium. Male albino rats were lactationally exposed to 0.2 % Pb-acetate or 0.2 % Pb-acetate together nutrient metal mixture (0.02 %) in drinking water of the mother from postnatal day 1 (PND1) to PND 21. The hemoglobin level, the activities of serum ceruloplasmin oxidase, cardiac mitochondrial enzymes catalase, manganese superoxide dismutase, copper zinc superoxide dismutase, glutathione peroxidase, succinate dehydrogenase, lipid peroxidation and Pb levels were analyzed at PND 45, 12 and 24 months age. The hematological parameters, and the cardiac TCA cycle and antioxidant enzyme markers and lipid peroxidation levels were significantly altered following Pb exposure in young rats (PND 45). These Pb induced changes persisted, though at much lower level in the aged rats. The Pb levels in blood and heart were also significantly higher in PND 45 and remained at detectable levels in older rats. The nutrient metal mixture containing iron, calcium and zinc significantly reversed these changes in all the chosen markers except lipid peroxidation in which the reversal effect was not significant. These data are supportive of age-related cardiac mitochondrial impairments and further provide evidence for the protective efficacy of nutrient metal mixture against Pb-toxicity.








Similar content being viewed by others
References
Basha R, Reddy GR (2010) Developmental exposure to lead and late life abnormalities of nervous system. Indian J Exp Biol 48:636–641
Basha MR, Wei W, Brydie M, Razmiafshari M, Zawia NH (2003) Lead–induced developmental perturbations in hippocampal Spl. DNA-binding are prevented by Zinc supplementation: in vivo evidence for Pb and Zn competition. Int J Dev Neurosci 21:1–12
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bernabucci U, Ronchi B, Lacetera N, Nardone A (2002) Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J Dairy Sci 85:2173–2179
Ceballos-picot I, Trivier JM, Nicole A, Sinet PM, Thevenin M (1992) Age-correlated modifications of copper-zinc superoxide dismutase and glutathione-related enzyme activities in human erythrocytes. Clin Chem 38:66–70
Cheng Y, Schwartz J, Vokonas PS, Weiss ST, Aro A, Hu H (1998) Electrocardiographic conduction disturbances in association with low-level lead exposure (the Normative Aging Study). Am J Cardiol 82:594–599
Davis JM, Svendsgaard DJ (1987) Lead and child development. Nature 329(6137):297–300
Flora SJS, Pande M, Kannan GM, Mehta A (2004) Lead induced oxidative stress and its recovery following co-administration of melatonin or N-acetylcysteine during chelation with succimer in male rats. Cell Mol Biol 50:543–551
Flora SJ, Saxena G, Mehta A (2007) Reversal of lead-induced neuronal apoptosis by chelation treatment in rats: role of reactive oxygen species and intracellular Ca(2+). J Pharmacol Exp Ther 322(1):108–116
Freieden E, Hsich HS (1976) Advances in Enzymology 44:893–896
Gottipolu RR, Wallenborn JG, Karoly ED, Schladweiler MC, Ledbetter AD, Krantz T, Linak WP, Nyska A, Johnson JA, Thomas R, Richards JE, Jaskot RH, Kodavanti UP (2009) One-month diesel exhaust inhalation produces hypertensive gene expression pattern in healthy rats. Environ Health Perspect 17:39–46
Guemouri L, Artur Y, Herbeth B, Jeandel C, Cuny G, Siets G (1991) Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin Chem 37:1932–1937
Harman D (1992) Role of free radicals in ageing and disease. Ann NY Acad Sci 673:598–620
Herenberg S, Nikkanen J (1972) Effect of lead on delta-aminolevulinic acid in the rat. Proc Soc Exp Biol Med 143:234–237
Herman DS, Geraldine M, Venkatesh T (2009) Influence of minerals on lead-induced alterations in liver function in rats exposed to long-term lead exposure. J Hazard Mater 166:1410–1414
Hipkiss A (2003) Errors, mitochondrial dysfunction and ageing. Biogerontology 4:397–400
Hiroshi O, Ohisi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by TBA reaction. Anal Biochem 95:351–358
Holliday R (2005) Streptomycin, errors in mitochondria and ageing. Biogerontology 6:431–432
Jacob B, Ritz B, Heinrich J, Hoelscher B, Wichmann HE (2000) The effect of low-level blood lead on hematologic parameters in children. Environ Res 82:150–159
Jegalian K (2005) Getting the lead out. http://www.nigms.nih.gov/news/findings/mar05/lead.html
Judge S, Leeuwenbugh C (2007) Cardiac mitochondrial bioenergetics, oxidative stress and aging. Am J Phydiol Cell Physiol 292:1983–1992
Kilikdar D, Mukherjee D, Mitra E, Ghosh KA, Basu A, Chandra MA, Bandyopdhyay D (2011) Protective effect of aqueous garlic extract against extract against lead-induced hepatic injury in rats. Ind J Exp Biol 49:498–510
Kim HS, Lee SS, Lee GS, Hwangbo Y, Ahn KD, Lee BK (2004) The protective effect of δ-aminolevulinic acid dehydratase 1-2 and 2-2 isozymes against blood lead with higher hematologic parameters. Environ Health Perspect 112:538–541
Korrick SA, Hunter DJ, Rotnitzky A, Hu H, Speizer FE (1999) Lead and hypertension in a sample of middle-aged women. Am J Public Health 89:330–335
Leelakunakorn W, Sriworawit R, Soontaros S (2005) Cerloplasmin oxidase activity as a biomarker of lead exposrure. J Occup Health 47:56–60
Liu MC, Zheng LY, Lu J, Fan HS, Wu MD, Ma QJ (2010) Quercetin protects rat liver against lead induced oxidative stress and apoptosis. Environ Toxicol Pharm 29:158–166
Liu MC, Ma QJ, Zhi Y (2011) Sun protective role of puerarin on lead-induced alterations of the hepatic glutathione antioxidant system and hyperlipidemia in rats. Food Chem Toxicol 49:3119–3127
Lowry OH, Rosenbrough NJ, Farr AL, Randal RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Lustberg M, Silbergeld E (2002) Blood lead levels and mortality. Arch Intern Med 162:2443–2449
Mahaffey KR (1981) Nutritional factors in lead poisoning. Nutr Rev 39:353–362
Mates JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Mylorie AA, Collins H, Umbles C, Kyle J (1986) Erythrocyte superoxide dismutase activity and other parameters of copper status in rats ingesting lead acetate. Toxicol Appl Pharmacol 82:512–520
Nachlas MM, Margulies SP, Seligman AM (1960) A colorimetric method for the estimation of succinic dehydrogenase activity. J Biol Chem 235:499–504
Nash D, Magder L, Lustberg M, Sherwin RW, Rubin RJ, Kaufmann RB, Silbergeld EK (2003) Blood lead, blood pressure, and hypertension in perimenopausal and postmenopausal women. JAMA 289(12):1523–1532
Oteiza PI, Verstraeten SV, Adonaylo VN (1995) Oxidative damage induced by metals without redox capacity in biological systems. Ci Cul 47:330–335
Petering HG (1980) The influence of dietary zinc and copper on the biologic effects of orally ingested lead in the rat. Ann NY Acad Sci 355:299–308
Prasanthi RPJ, Reddy GH, Devi CB, Reddy GR (2005) Zinc and calcium reduce lead induced perturbations in the aminergic system of developing rat brain. Biometals 18:615–626
Prasanthi RPJ, Devi CB, Basha DC, Reddy NS, Reddy GR (2010) Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int J Devl Neurosci 28:161–167
Preuss HG (1993) A review of persistent, low-grade lead challenge: neurological and cardiovascular consequences. J Am Coll Nutr 12:246–254
Rattan SIS (2006) Theories of biological aging: genes, proteins, and free radicals. Free Radical Res 40(12):1230–1238
Reddy GR, Zawia NH (2000) Lead exposure alters Egr-1 DNA binding in the neonatal rat brain. Int J Dev Neurosci 18:791–795
Reddy GR, Devi CB, Chetty CS (2007) Developmental lead neurotoxicity: alterations in brain cholinergic system. NeuroToxicology 28:402–407
Sastre J, Pallardo FV, Vina J (2003) The role of mitochondrial oxidative stress in aging. Free Radical Biol Med 35:1–8
Schosinsky KH, Lehmann HP, Beeler MF (1974) Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin Chem 20:1556–1563
Sitte N, von Zglinicki T (2003) Free radical production and antioxidant defense: a primer. In: von Zglinicki T (ed) Aging at the molecular level. Kluwer Academy Publisher, Dordrecht, pp 1–10
Sivaprasad R, Nagaraj M, Varalakshmi P (2003) Combined efficacies of lipoic acid and meso-2,3-dimercaptosuccinic acid on lead-induced erythrocyte membrane lipid peroxidation and antioxidant status in rats. Jum Exp Toxicol 22:183–192
Suh JH, Health S, Hagen TM (2003) Two subpopulations of mitochondria in the aging rat heart display heterogeneous levels of oxidative stress. Free Radic Bio Med 35:1064–1072
Szynaka B, Andrzejewska A, Tomasiak M, Augustynowicz A (1999) Exocrine cell mitochondria of the rat pancreas after lead intoxication. Exp Toxicol Pathol 51(6):559–564
Tappel ME, Chaudiere J, Tappel AL (1982) Glutathione peroxidase activities of animal tissues. Comp Biochem Physiol 73:945–949
Vargas I, Castillo C, Posadas F, Escalante B (2003) Acute lead exposure induces renal heme oxygenase-1 and decreases urinary Na+ excretion. Hum Exp Toxicol 22:237–244
Vaziri ND (2002) Pathogenesis of lead-induced hypertension: role of oxidative stress. J Hypertens Suppl 20:S15–S20
Wang Q, Luo W, Zheng W, Liu Y, Xu H, Zheng G, Dai Z, Zang W, Chen Y, Chen J (2007) Iron supplement prevents lead-induced disruption of the blood-brain barrier during rat development. Toxicol Appl Pharmacol 219:33–41
Ziegler EE, Edwards BB, Tensen RL, Mahaffey KR, Forman SI (1978) Absorption and retention of lead by infants. Pediatr Res 12:29–34
Zimmermann MB, Muthayya S, Moretti D, Kurpad A, Hurrell RF (2006) Iron fortification reduces blood lead levels in children in Bangalore, India. Pediatrics 117:2014–2021
Acknowledgments
This study was supported by Council of Scientific and Industrial Research (CSIR), grant No. 37 (1349)/08/EMR-II.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Basha, D.C., Basha, S.S. & Reddy, G.R. Lead-induced cardiac and hematological alterations in aging Wistar male rats: alleviating effects of nutrient metal mixture. Biogerontology 13, 359–368 (2012). https://doi.org/10.1007/s10522-012-9380-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-012-9380-9