Abstract
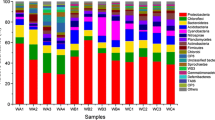
The retention of aquatic plant debris in freshwater systems favors a reduction in soluble reactive phosphorus (P) in overlying water through microbe-mediated mechanisms in sediment. For a more complete view of the changes in sediment microbial structure and functioning when receiving plant debris, the enzyme activities and microbial community structure in sediments incubated with or without plant debris were investigated. Significantly higher fluorescein diacetate (FDA) hydrolysis, alkaline phosphatase, polyphenol oxidase, cellulase, β-glucosidase, and dehydrogenase activities were observed with plant debris treatment. High-throughput pyrosequencing showed that the number of total operational taxonomic units (OTUs) of bacteria estimated by using the Chao1 analysis was 2064 (in the control) and 1821 (with the plant debris treatment). The Shannon index, functional organization, and Venn diagrams revealed that the enriched OTUs in plant debris-treated community were less diversified than those in the control sample. The prominent bacterial phyla Firmicutes and Bacteroidetes were more diverse after plant debris addition. At the class level, the relative abundance of Alphaproteobacteria increased by 114% when plant debris was added, whereas the relative abundances of Beta-, Delta-, and Gammaproteobacteria decreased by 42, 78, and 86%, respectively. Azospirillum and Dechloromonas, the dominant phylogenetic groups at the genus level, increased with plant debris addition. Our study showed the importance of the above microbial genera in plant debris-mediated P retention in sediment.






Similar content being viewed by others
References
Acosta-Martínez V, Dowd SE, Sun Y, Wester D, Allen V (2010) Pyrosequencing analysis for characterization of soil bacterial populations as affected by an integrated livestock-cotton production system. Appl Soil Ecol 45:13–25
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33:943–951
Ahn JH, Lee SA, Kim JM, Kim MS, Song J, Weon HY (2016) Dynamics of bacterial communities in rice field soils as affected by different long-term fertilization practices. J Microbiol 54:724–731
An WC, Li XM (2009) Phosphate adsorption characteristics at the sediment–water interface and phosphorus fractions in Nansi Lake, China, and its main inflow rivers. Environ Monit Assess 148:1–4
Badiane NNY, Chotte JL, Pate E, Masse D, Rouland C (2001) Use of soil enzyme activities to monitor soil quality in natural and improved fallows in semi-arid tropical regions. Appl Soil Ecol 18:229–238
Bai YH, Shi Q, Wen DH, Li ZX, Jefferson WA, Feng CP, Tang XY (2012) Bacterial communities in the sediments of dianchi lake, a partitioned eutrophic waterbody in China. PLoS One 7:e37796
Bao SD (2005) Soil and agricultural chemistry analysis. Chinese Agricultural Publishing House, Beijing in Chinese
Berlemont R, Allison SD Weihe C, Lu Y, Brodie EL, Martiny JBH, Martiny AC (2014) Cellulolytic potential under environmental changes in microbial communities from grassland litter. Front Microbiol 5:639
Casida LE Jr, Klein DA, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98:371–376
Chen N, Yang JS, Qu JH, Li HF, Liu WJ, Li BZ, Wang WT, Yuan HL (2015a) Sediment prokaryote communities in different sites of eutrophic Lake Taihu and their interactions with environmental factors. World J Microbiol Biotechnol 31:883–896
Chen Y, Wen Y, Tang ZR, Huang JG, Zhou Q, Vymazal J (2015b) Effects of plant biomass on bacterial community structure in constructed wetlands used for tertiary wastewater treatment. Ecol Eng 84:38–45
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens GE (2009) Controlling eutrophication: nitrogen and phosphorus. Science 323:1014–1015
Dai J, Chen D, Gao G, Tang X, Wu S, Wu X, Zhou J (2014) Recovery of novel alkaline phosphatase-encoding genes (phoX) from eutrophic Lake Taihu. Can J Microbiol 60:167–171
Davelaar D (1993) Ecological significance of bacterial polyphosphate metabolism in sediments. Hydrobiologia 253:179–192
DeBusk WF, Reddy KR (2005) Litter decomposition and nutrient dynamics in a phosphorus enriched everglades marsh. Biogeochemistry 75:217–240
Du ST, Shentu JL, Luo BF, Shamsi IH, Lin XY, Zhang YS, Jin CW (2011) Facilitation of phosphorus adsorption onto sediment by aquatic plant debris. J Hazard Mater 191:212–218
Eivazi F, Tabatabai MA (1988) Glucosidases and galactosidases in soil. Soil Biol Biochem 20:601–606
Fales FW (1951) The assimilation and degradation of carbohydrates by yeast cells. J Biol Chem 193:113–124
Fan CX, Hu WP, Phillip WF, Chen YW, Qu WC, Zhang L (2005) Carbon dioxide partial pressure and carbon fluxes of air-water interface in Lake Taihu, China. Chin J Oceanol Limnol 23:29–38
Foulquier A, Mermillod-Blondin F, Malard F, Gibert J (2011) Response of sediment biofilm to increased dissolved organic carbon supply in groundwater artificially recharged with stormwater. J Soils Sediments 11:382–393
Gächter R, Meyer JS (1993) The role of microorganisms in mobilization and fixation of phosphorus in sediments. Hydrobiologia 253:103–121
Gächter R, Meyer JS, Mares A (1988) Contribution of bacteria to release and fixation of phosphorus in lake sediments. Limnol Oceanogr 33:1542–1558
Goel RK, Sanhueza P, Noguera DR (2005) Evidence of Dechloromonas sp. participating in enhanced biological phosphorus removal (EBPR) in a bench-scale aerated-anoxic reactor. P Water Environ Fed 12:3864–3871
Goffredi SK, Orphan VJ (2010) Bacterial community shifts in taxa and diversity in response to localized organic loading in the deep sea. Environ Microbiol 12:344–363
Graham EB, Knelman JE, Gabor RS, Schooler S, McKnight DM, Nemergut D (2016) Dissolved organic matter and inorganic mercury loadings favor novel methylators and fermentation metabolisms in oligotrophic sediments. bioRxiv, doi: 10.1101/072017
He SM, McMahon KD (2011) ‘Candidatus Accumulibacter’ gene expression in response to dynamic EBPR conditions. ISME J 5:329–340
Hibbing ME, Fuqua C, Parsek MR, Peterson SB (2010) Bacterial competition: surviving in the microbial jungle. Nat Rev Microbiol 8:15–25
Huang LD, Du ST, Fan L, Lin XY, Wang HL, Zhang YS (2011) Microbial activity facilitates phosphorus adsorption to shallow lake sediment. J Soils Sediments 11:185–193
Hupfer M, Gächter R, Rüegger H (1995) Poly-P in lake sediments. 31P-NMR spectroscopy as a tool for its identification. Limnol Oceanogr 40:610–617
Hupfer M, Gloess S, Grossart HP (2007) Polyphosphate-accumulating microorganisms in aquatic sediments. Aquat Microb Ecol 47:299–311
Jansson M, Olsson H, Pettersson K (1988) Phosphatases; origin, characteristics and function in lakes. Hydrobiologia 170:157–175
Jeppesen E, Jensen JP, Søndergaard M, Lauridsen T (1999) Trophic dynamics in turbid and Clearwater lakes with special emphasis on the role of zooplankton for water clarity. Hydrobiologia 408:217–231
Kawaharasaki M, Tanaka H, Kanagawa T, Nakamura K (1999) In situ identification of polyphosphate-accumulating bacteria in activated sludge by dual staining with rRNAtargeted oligonucleotide probes and 4′,6-diamidino-2-phenylindol (DAPI) at a polyphosphate-probing concentration. Water Res 33:257–265
Khoshmanesh A, Hart BT, Duncan A, Beckett R (2002) Luxury uptake of phosphorus by sediment bacteria. Water Res 36:774–778
Lan Y, Cui BS, You ZY, Li X, Han Z, Zhang YT, Zhang Y (2012) Litter decomposition of six macrophytes in a eutrophic shallow lake (Baiyangdian Lake, China). Clean-Soil Air Water 40:1159–1166
Li D, Alidina M, Ouf M, Sharp JQ, Saikaly P, Drewes JE (2013) Microbial community evolution during simulated managed aquifer recharge in response to different biodegradable dissolved organic carbon (BDOC) concentrations. Water Res 47:2421–2430
Lürling M, Oosterhout FV (2013) Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Res 47:6527–6537
Ma J, Wang Z, Yang Y, Mei X, Wu Z (2013) Correlating microbial community structure and composition with aeration intensity in submerged membrane bioreactors by 454 high-throughput pyrosequencing. Water Res 47:859–869
Marzorati M, Wittebolle L, Boon N, Daffonchio D, Verstraete W (2008) How to get more out of molecular fingerprints: practical tools for microbial ecology. Environ Microbiol 10:1571–1581
Meza B, de-Bashan LE, Hernandez JP, Bashan Y (2015) Accumulation of intra-cellular polyphosphate in Chlorella vulgaris cells is related to indole-3-acetic acid produced by Azospirillum brasilense. Res Microbiol 166:399–407
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural water. Anal Chim Acta 27:31–36
Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S (2011) A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75:14–49
Newton RJ, McMahon KD (2011) Seasonal differences in bacterial community composition following nutrient additions in a eutrophic lake. Environ Microbiol 13:887–899
Perucci P, Casucci C, Dumontet S (2000) An improved method to evaluate the o-diphenol oxidase activity of soil. Soil Biol Biochem 32:1927–1933
Ramm K, Scheps V (1997) Phosphorus balance of a polytrophic shallow lake with the consideration of phosphorus release. Hydrobiologia 342(343):43–53
Reynolds CS (1996) Phosphorus recycling in lakes: evidence from large limnetic enclosures for the importance of shallow sediments. Freshw Biol 35:623–645
Rui J, Peng J, Lu Y (2009) Succession of bacterial populations during plant residue decomposition in rice field soil. Appl Environ Microbiol 75:4879–4886
Santibáñez VV (2009) The role of microbial processes in soil phosphorus dynamics, Cornell University
Sanyal A, Antony R, Samui G, Thamban M (2018) Microbial communities and their potential for degradation of dissolved organic carbon in cryoconite hole environments of Himalaya and Antarctica. Microbiol Res 208:32–42. https://doi.org/10.1016/j.micres.2018.01.004
Schumacher TE, Eynard A, Chintala R (2015) Rapid cost-effective analysis of microbial activity in soils using modified fluorescein diacetate method. Environ Sci Pollut Res 22:4759–4762
Shannon CR, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Sinsabaugh RL, Hill BH, Shah JJF (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798
Sinsabaugh RL, Shah JJF (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43:313–343
Sinsabaugh RL, Shah JJF, Hill BH (2012) Ecoenzymatic stoichiometry of stream sediments with comparison to terrestrial soils. Biogeochemistry 111:455–467
Smith VH, Tilman GD, Nekol JC (1999) Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ Pollut 100:179–196
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tang XM, Gao G, Qin B, Zhu LP, Chao JY, Wang JJ, Yang GJ (2009) Characterization of bacterial communities associated with organic aggregates in a large, shallow, eutrophic freshwater lake (Lake Taihu, China). Microb Ecol 58:307–322
Uhlmann D, Bauer HD (1988) A remark on microorganisms in lake-sediments with emphasis on polyphosphate-accumulating bacteria. Int Rev Hydrobiol 73:703–708
Valiela I, Teal JM, Allen SD, Van Etten R, Goehringer D, Volkmann S (1985) Decomposition in salt marsh ecosystems: the phases and major factors affecting the disappearance of above-ground organic matter. J Exp Mar Biol Ecol 89:29–54
Van Soest PJ (1963) Use of detergents in analysis of fibrous feeds II. A rapid method for determination of fiber and lignin. J Assoc Off Agr Chem 46:829–835
Xie YH, Yu D, Ren B (2004) Effects of nitrogen and phosphorus availability on the decomposition of aquatic plants. Aquat Bot 80:29–37
Xiong W, Xie P, Wang SR, Niu Y, Yang X, Chen WJ (2015) Sources of organic matter affect depth-related microbial community composition in sediments of Lake Erhai, Southwest China. J Limnol 74:310–323
Zamparas M, Zacharias L (2014) Restoration of eutrophic freshwater by managing internal nutrient loads. A review. Sci Total Environ 496:551–562
Acknowledgments
This work was supported by the Science and Technology Department Commonwealth Technology Applied Research Project of Zhejiang Province (2017C32001) and the Student’s Science and Technology Innovation Project (2017R408056).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Xue, WL., Pan, W., Lu, Q. et al. Aquatic plant debris changes sediment enzymatic activity and microbial community structure. Environ Sci Pollut Res 25, 21801–21810 (2018). https://doi.org/10.1007/s11356-018-2310-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2310-x