Abstract
Purpose
Overexpression and activation of matrix metalloproteinase-13 (MMP-13) within atheroma increases susceptibility to plaque rupture, a major cause of severe cardiovascular complications. In comparison to pan-MMP targeting [18F]BR-351, we evaluated the potential for [18F]FMBP, a selective PET radiotracer for MMP-13, to detect extracellular matrix (ECM) remodeling in vascular plaques possessing markers of inflammation.
Procedures
[18F]FMBP and [18F]BR-351 were initially assessed in vitro by incubation with en face aortae from 8 month-old atherogenic ApoE−/− mice. Ex vivo biodistributions, plasma metabolite analyses, and ex vivo autoradiography were analogously performed 30 min after intravenous radiotracer administration in age-matched C57Bl/6 and ApoE−/− mice under baseline or homologous blocking conditions. En face aortae were subsequently stained with Oil Red O (ORO), sectioned, and subject to immunofluorescence staining for Mac-2 and MMP-13.
Results
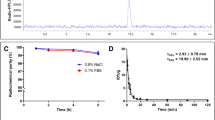
High-resolution autoradiographic image analysis demonstrated target specificity and regional concordance to lipid-rich lesions. Biodistribution studies revealed hepatobiliary excretion, low accumulation of radioactivity in non-excretory organs, and few differences between strains and conditions in non-target organs. Plasma metabolite analyses uncovered that [18F]FMBP exhibited excellent in vivo stability (≥74% intact) while [18F]BR-351 was extensively metabolized (≤37% intact). Ex vivo autoradiography and histology of en face aortae revealed that [18F]FMBP, relative to [18F]BR-351, exhibited 2.9-fold greater lesion uptake, substantial specific binding (68%), and improved sensitivity to atherosclerotic tissue (2.9-fold vs 2.1-fold). Immunofluorescent staining of aortic en face cross sections demonstrated elevated Mac-2 and MMP-13-positive areas within atherosclerotic lesions identified by [18F]FMBP ex vivo autoradiography.
Conclusions
While both radiotracers successfully identified atherosclerotic plaques, [18F]FMBP showed superior specificity and sensitivity for lesions possessing features of destructive plaque remodeling. The detection of ECM remodeling by selective targeting of MMP-13 may enable characterization of high-risk atherosclerosis featuring elevated collagenase activity.





Similar content being viewed by others
References
Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15:786–801
Löffek S, Schilling O, Franzke C-W (2011) Biological role of matrix metalloproteinases: a critical balance. Eur Respir J 38:191–208
Li H, Wang D, Yuan Y, Min J (2017) New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther 19:248
Hu J, Van den Steen PE, Sang Q-XA, Opdenakker G (2007) Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov 6:480–498
Johnson JL (2017) Metalloproteinases in Atherosclerosis. Eur J Pharmacol 816:93–106
Giannakou S, Angelidis G, Tsougos I, Valotassiou V, Kappas K, Georgoulias P (2020) Pet tracers for vulnerable plaque imaging. Ann Nucl Med 34:305–313
Heo R, Nakazato R, Kalra D, Min JK (2014) Noninvasive imaging in coronary artery disease. Semin Nucl Med 44:398–409
Owen DRJ, Lindsay AC, Choudhury RP, Fayad ZA (2011) Imaging of atherosclerosis. Annu Rev Med 62:25–40
Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874
Sukhova GK, Schönbeck U, Rabkin E et al (1999) Evidence for Increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation 99:2503–2509
Lessner SM, Galis ZS (2004) Matrix metalloproteinases and vascular endothelium-mononuclear cell close encounters. Trends Cardiovasc Med 14:105–111
Newby AC (2012) Matrix metalloproteinase inhibition therapy for vascular diseases. Vasc Pharmacol 56:232–244
Schaar J (2004) Terminology for high-risk and vulnerable coronary artery plaques. Eur Heart J 25:1077–1082
Newby AC (2014) Proteinases and plaque rupture: unblocking the road to translation. Curr Opin Lipidol 25:358–366
Quillard T, Araújo HA, Franck G, Tesmenitsky Y, Libby P (2014) Matrix metalloproteinase-13 predominates over matrix metalloproteinase-8 as the functional interstitial collagenase in mouse atheromata. Arterioscler Thromb Vasc Biol 34:1179–1186
Quillard T, Tesmenitsky Y, Croce K, Travers R, Shvartz E, Koskinas KC, Sukhova GK, Aikawa E, Aikawa M, Libby P (2011) Selective inhibition of matrix metalloproteinase-13 Increases collagen content of established mouse Atherosclerosis. Arterioscler Thromb Vasc Biol 31:2464–2472
Deguchi J, Aikawa E, Libby P et al (2005) Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation 112:2708–2715
Vazquez N, Missault S, Vangestel C, Deleye S, Thomae D, van der Veken P, Augustyns K, Staelens S, Dedeurwaerdere S, wyffels L (2017) Evaluation of [18F]BR420 and [18F]BR351 as radiotracers for MMP-9 imaging in colorectal cancer. J Label Compd Radiopharm 60:69–79
Ye Y, Toczek J, Gona K, Kim HY, Han J, Razavian M, Golestani R, Zhang J, Wu TL, Ghosh M, Jung JJ, Sadeghi MM (2018) Novel arginine-containing macrocyclic MMP inhibitors: synthesis, 99mTc-labeling, and evaluation. Sci Rep 8:11647
auf dem Keller U, Bellac CL, Li Y et al (2010) Novel matrix metalloproteinase inhibitor [18F]marimastat-aryltrifluoroborate as a probe for in vivo positron emission tomography imaging in cancer. Cancer Res 70:7562–7569
Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ (2000) A Matrix Metalloproteinase Induction/Activation System Exists in the Human Left Ventricular Myocardium and Is Upregulated in Heart Failure. Circulation 102:1944–1949
Hugenberg V, Wagner S, Kopka K, Schäfers M, Schuit RC, Windhorst AD, Hermann S (2017) Radiolabeled Selective matrix metalloproteinase 13 (MMP-13) inhibitors: (radio)syntheses and in vitro and first in vivo evaluation. J Med Chem 60:307–321
Nara H, Sato K, Naito T, Mototani H, Oki H, Yamamoto Y, Kuno H, Santou T, Kanzaki N, Terauchi J, Uchikawa O, Kori M (2014) Discovery of novel, highly potent, and selective quinazoline-2-carboxamide-based matrix metalloproteinase (MMP)-13 inhibitors without a zinc binding group using a structure-based design approach. J Med Chem 57:8886–8902
Rangasamy L, Di Geronimo B, Ortín I et al (2019) Molecular Imaging probes based on matrix metalloproteinase inhibitors (MMPIs). Molecules 24:2982
Engel CK, Pirard B, Schimanski S, Kirsch R, Habermann J, Klingler O, Schlotte V, Weithmann KU, Wendt KU (2005) Structural basis for the highly selective inhibition of MMP-13. Chem Biol 12:181–189
Wagner S, Breyholz H-J, Law MP, Faust A, Höltke C, Schröer S, Haufe G, Levkau B, Schober O, Schäfers M, Kopka K (2007) Novel fluorinated derivatives of the broad-spectrum MMP inhibitors N -hydroxy-2( R )-[[(4-methoxyphenyl)sulfonyl](benzyl)- and (3-picolyl)-amino]-3-methyl-butanamide as potential tools for the molecular imaging of activated MMPs with PET. J Med Chem 50:5752–5764
Zinnhardt B, Pigeon H, Thézé B, Viel T, Wachsmuth L, Fricke IB, Schelhaas S, Honold L, Schwegmann K, Wagner S, Faust A, Faber C, Kuhlmann MT, Hermann S, Schäfers M, Winkeler A, Jacobs AH (2017) Combined PET imaging of the inflammatory tumor microenvironment identifies margins of unique radiotracer uptake. Cancer Res 77:1831–1841
Zinnhardt B, Viel T, Wachsmuth L, Vrachimis A, Wagner S, Breyholz HJ, Faust A, Hermann S, Kopka K, Faber C, Dollé F, Pappata S, Planas AM, Tavitian B, Schäfers M, Sorokin LM, Kuhlmann MT, Jacobs AH (2015) Multimodal imaging reveals temporal and spatial microglia and matrix metalloproteinase activity after experimental stroke. J Cereb Blood Flow Metab 35:1711–1721
Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R (1994) ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb Vasc Biol 14:133–140
Andrés-Manzano MJ, Andrés V, Dorado B (2015) Oil Red O and hematoxylin and eosin staining for quantification of atherosclerosis burden in mouse aorta and aortic root. Methods Mol Biol Clifton 1339:85–99
Karunakaran D, Geoffrion M, Wei L, Gan W, Richards L, Shangari P, DeKemp EM, Beanlands RA, Perisic L, Maegdefessel L, Hedin U, Sad S, Guo L, Kolodgie FD, Virmani R, Ruddy T, Rayner KJ (2016) Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv 2:e1600224
Ohshima S, Petrov A, Fujimoto S, Zhou J, Azure M, Edwards DS, Murohara T, Narula N, Tsimikas S, Narula J (2009) Molecular imaging of matrix metalloproteinase expression in atherosclerotic plaques of mice deficient in apolipoprotein E or low-density-lipoprotein receptor. J Nucl Med 50:612–617
Tavakoli S, Razavian M, Zhang J, Nie L, Marfatia R, Dobrucki LW, Sinusas AJ, Robinson S, Edwards DS, Sadeghi MM (2011) Matrix metalloproteinase activation predicts amelioration of remodeling after dietary modification in injured arteries. Arterioscler Thromb Vasc Biol 31:102–109
Zhang J, Nie L, Razavian M, Ahmed M, Dobrucki LW, Asadi A, Edwards DS, Azure M, Sinusas AJ, Sadeghi MM (2008) Molecular imaging of activated matrix metalloproteinases in vascular remodeling. Circulation 118:1953–1960
Razavian M, Tavakoli S, Zhang J, Nie L, Dobrucki LW, Sinusas AJ, Azure M, Robinson S, Sadeghi MM (2011) Atherosclerosis plaque heterogeneity and response to therapy detected by in vivo molecular imaging of matrix metalloproteinase activation. J Nucl Med 52:1795–1802
Fujimoto S, Hartung D, Ohshima S, Edwards DS, Zhou J, Yalamanchili P, Azure M, Fujimoto A, Isobe S, Matsumoto Y, Boersma H, Wong N, Yamazaki J, Narula N, Petrov A, Narula J (2008) Molecular imaging of matrix metalloproteinase in atherosclerotic lesions: resolution with dietary modification and statin therapy. J Am Coll Cardiol 52:1847–1857
Schäfers M, Riemann B, Kopka K et al (2004) Scintigraphic imaging of matrix metalloproteinase activity in the arterial wall in vivo. Circulation 109:2554–2559
Kiugel M, Hellberg S, Käkelä M, Liljenbäck H, Saanijoki T, Li XG, Tuomela J, Knuuti J, Saraste A, Roivainen A (2018) Evaluation of [68Ga]Ga-DOTA-TCTP-1 for the detection of metalloproteinase 2/9 expression in mouse atherosclerotic plaques. Molecules 23:3168
Veseli BE, Perrotta P, De Meyer GRA et al (2017) Animal models of atherosclerosis. Eur J Pharmacol 816:3–13
Müller A, Krämer SD, Meletta R, Beck K, Selivanova SV, Rancic Z, Kaufmann PA, Vos B, Meding J, Stellfeld T, Heinrich TK, Bauser M, Hütter J, Dinkelborg LM, Schibli R, Ametamey SM (2014) Gene expression levels of matrix metalloproteinases in human atherosclerotic plaques and evaluation of radiolabeled inhibitors as imaging agents for plaque vulnerability. Nucl Med Biol 41:562–569
Newby AC (2007) Metalloproteinases and vulnerable atherosclerotic plaques. Trends Cardiovasc Med 17:253–258
Schierwagen R, Maybüchen L, Zimmer S et al (2015) Seven weeks of western diet in apoliprotein-E-deficient mice induce metabolic syndrome and non-alcoholic steatohepatitis with liver fibrosis. Sci Rep 5:12931
Duarte S, Baber J, Fujii T, Coito AJ (2015) Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol 44-46:147–156
Dalvie D, Cosker T, Boyden T, Zhou S, Schroeder C, Potchoiba MJ (2008) Metabolism distribution and excretion of a matrix metalloproteinase-13 inhibitor, 4-[4-(4-fluorophenoxy)-benzenesulfonylamino]tetrahydropyran-4-carboxylic acid hydroxyamide (CP-544439), in rats and dogs: assessment of the metabolic profile of CP-544439 in plasma and urine of humans. Drug Metab Dispos 36:1869–1883
Kuzuya M, Nakamura K, Sasaki T, Wu Cheng X, Itohara S, Iguchi A (2006) Effect of MMP-2 deficiency on atherosclerotic lesion formation in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 26:1120–1125
Johnson JL, George SJ, Newby AC, Jackson CL (2005) Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A 102:15575–15580
Van Wart HE, Birkedal-Hansen H (1990) The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A 87:5578–5582
Jackson CL (2007) Defining and defending murine models of plaque rupture. Arterioscler Thromb Vasc Biol 27:973–977
Acknowledgements
We are grateful to Dr. Rob Beanlands and Dr. Katey Rayner for illuminating discussions, Dr. Tayebeh Hadizad and Daniel Duan for isotope production, and the University of Ottawa Heart Institute Animal Care and Veterinary Services for their contributions to this work.
Funding
The authors receive financial support from CIHR Project Grant 366633, CFI JELF 36848, Ontario Ministry of Research Innovation and Science ER17-13-119, and the Faculty of Medicine and Division of Cardiology at University of Ottawa. A.B. was supported by NSERC USRA and OGS. G.F. was supported by CIHR CGSM and QEII-GSST.
Author information
Authors and Affiliations
Contributions
Project design—A.B. and B.H.R.
Method development—A.B., M.M., G.F., X.Z., and R.A-H.
Data acquisition and analysis—A.B., M.M., G.F., and E.F.
Writing and revision of the manuscript—A.B., M.M., G.F., and B.H.R.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 909 kb)
Rights and permissions
About this article
Cite this article
Buchler, A., Munch, M., Farber, G. et al. Selective Imaging of Matrix Metalloproteinase-13 to Detect Extracellular Matrix Remodeling in Atherosclerotic Lesions. Mol Imaging Biol 24, 93–103 (2022). https://doi.org/10.1007/s11307-021-01626-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01626-9