Abstract
Purpose
P2X7 receptors have been considered as a promising biomarker for vulnerable atherosclerotic plaques, which are highly expressed by that instability-associated factors such as macrophages. Thus, we aim to investigate the feasibility of using specific P2X7-targeted 18F-labeled tracer 18F-FTTM ((2-chloro-3-[18F]fluorophenyl)[1,4,6,7-tetrahydro-1-(2-pyrimidinyl)-5H-1,2,3-triazolo[4,5-c]pyridin-5-yl]methanone) for PET study of vulnerable atherosclerotic plaques identification.
Method
The radioligand 18F-FTTM was achieved based on the copper-mediated radiofluorination of arylstannane. In vitro and in vivo experiments were performed to verify the biochemical properties. Dynamic 18F-FTTM Micro-PET/CT imaging was performed for 1 h on ApoE−/− mice (10, 20, 30 weeks on high-fat diet) and wild-type C57BL/6 J mice on normal diet. Ex vivo PET imaging was conducted to verify the specificity of the radioligand. Serum inflammatory cytokines, lipids, and lipoproteins profiles were detected by ELISA. The lipid distribution and morphology of plaques were evaluated by Oil Red O, HE, Masson, and immunofluorescence stainings.
Results
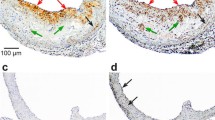
18F-FTTM was afforded with decay-corrected radiochemical yields of 5–10%, specific activity of 269–320 MBq/nmol (n = 8, EOS), and radiochemical purity of above 99%. 18F-FTTM showed excellent stability in vitro, rapid blood clearance in mice, good affinity to RAW264.7 cells. We observed an increase in both in vivo and ex vivo imagings as disease progressed, and the imaging signatures correlated with histopathological features. Furthermore, compared with 18F-FDG imaging, the SUVmax values of 18F-FTTM at the aortic arch of ApoE−/− mice of high-fat feeding for 20 and 30 weeks were 43% and 53% higher than those of the control group, respectively.
Conclusion
We innovatively apply a new type P2X7-targeted PET probe (18F-FTTM) to identify vulnerable atherosclerotic plaques, to detect the inflammatory response of atherosclerosis, and to provide a powerful non-invasive method for the diagnosis of atherosclerotic lesions and new drug screening for accurate treatment.





Similar content being viewed by others
References
Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–72.
Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8.
Libby P, DiCarli M, Weissleder R. The vascular biology of atherosclerosis and imaging targets. J Nucl Med. 2010;51(Suppl 1):33s–7s.
Tarkin JM, Dweck MR, Evans NR, et al. Imaging Atherosclerosis. Circ Res. 2016;118:750–69.
Tarkin JM, Joshi FR, Rudd JH. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol. 2014;11:443–57.
Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet (London, England). 2014;383:705–13.
Dweck MR, Chow MW, Joshi NV, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59:1539–48.
Jiang L, Tu Y, Kimura RH, et al. 64Cu-Labeled Divalent Cystine Knot Peptide for Imaging Carotid Atherosclerotic Plaques. J Nucl Med. 2015;56:939–44.
Nie X, Laforest R, Elvington A, et al. PET/MRI of Hypoxic Atherosclerosis Using 64Cu-ATSM in a Rabbit Model. J Nucl Med. 2016;57:2006–11.
MacAskill MG, Newby DE, Tavares AAS. Frontiers in positron emission tomography imaging of the vulnerable atherosclerotic plaque. Cardiovasc Res. 2019;115:1952–62.
Shokoples BG, Paradis P, Schiffrin EL. P2X7 Receptors: An Untapped Target for the Management of Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2021;41:186–99.
Peng K, Liu L, Wei D, et al. P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int J Mol Med. 2015;35:1179–88.
Piccini A, Carta S, Tassi S, Lasiglié D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–72.
Danquah W, Meyer-Schwesinger C, Rissiek B, et al. Nanobodies that block gating of the P2X7 ion channel ameliorate inflammation. Sci transl med. 2016;8:366ra162.
Piscopiello M, Sessa M, Anzalone N, et al. P2X7 receptor is expressed in human vessels and might play a role in atherosclerosis. Int J Cardiol. 2013;168:2863–6.
Stachon P, Heidenreich A, Merz J, et al. P2X7 Deficiency Blocks Lesional Inflammasome Activity and Ameliorates Atherosclerosis in Mice. Circulation. 2017;135:2524–33.
Savio LEB, de Andrade MP, da Silva CG, Coutinho-Silva R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front Pharmacol. 2018;9:52.
Ridker PM, Howard CP, Walter V, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–48.
Lombardi M, Mantione ME, Baccellieri D, et al. P2X7 receptor antagonism modulates IL-1β and MMP9 in human atherosclerotic vessels. Sci Rep. 2017;7:4872.
Burnstock G. Purinergic Signaling in the Cardiovascular System. Circ Res. 2017;120:207–28.
Chen Z, He L, Li L, Chen L. The P2X7 purinergic receptor: An emerging therapeutic target in cardiovascular diseases. Clin Chim Acta. 2018;479:196–207.
Zhang L, Hu K, Shao T, et al. Recent developments on PET radiotracers for TSPO and their applications in neuroimaging. Acta pharmaceutica Sinica B. 2021;11:373–93.
Fu Z, Lin Q, Hu B, et al. P2X7 PET Radioligand 18F-PTTP for Differentiation of Lung Tumor from Inflammation. J Nucl Med. 2019;60:930–6.
Zheng QH. Radioligands targeting purinergic P2X7 receptor. Bioorg med chem lett. 2020;30:127169.
Savall BM, Wu D, De Angelis M, et al. Synthesis, SAR, and Pharmacological Characterization of Brain Penetrant P2X7 Receptor Antagonists. ACS Med Chem Lett. 2015;6:671–6.
McCluskey SP, Plisson C, Rabiner EA, Howes O. Advances in CNS PET: the state-of-the-art for new imaging targets for pathophysiology and drug development. Eur J Nucl Med Mol Imaging. 2020;47:451–89.
Nakashima Y, Plump AS, Raines EW, et al. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–40.
Plump AS, Smith JD, Hayek T, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–53.
Plump AS, Breslow JL. Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu Rev Nutr. 1995;15:495–518.
Emini Veseli B, Perrotta P, De Meyer GRA, et al. Animal models of atherosclerosis. Eur J Pharmacol. 2017;816:3–13.
Funding
This study was funded by the National Nature Science Foundation of China (No.81471706, 11875114, 81871407, China), Shanghai Municipal Science and Technology Committee of Shanghai outstanding academic leaders plan (No.21XD1423500, China), Shanghai Municipal Key Clinical Specialty Project (SHSLCZDZK03401, China) and Clinical Research Project of Zhongshan Hospital(No.2020ZSLC20, China).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Animal care and experimental procedures were conducted in compliance with the Helsinki Declaration and approved by the Ethical Committee of Zhongshan Hospital, Fudan University.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, Z., Lin, Q., Xu, Z. et al. P2X7 receptor-specific radioligand 18F-FTTM for atherosclerotic plaque PET imaging. Eur J Nucl Med Mol Imaging 49, 2595–2604 (2022). https://doi.org/10.1007/s00259-022-05689-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05689-w