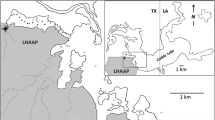
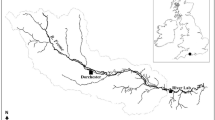
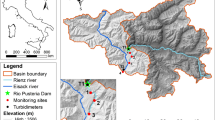
Abstract
Agricultural land use is widely accepted to elicit changes on surrounding environment and neighboring ecosystems. Meanwhile, the impact of different types of agricultural land use likely cause a variety of impacts on nearby ecosystems and the organisms that inhabit them. Freshwater systems support a wide range of organisms—from infaunal or epifaunal invertebrates to mobile pelagic and littoral fish species. The focus of this study was to determine how agricultural activity in the upstream catchment influences sediment properties and the resulting ability of three distinct invertebrate species to survive and reproduce in these different sediments. This will be the first study that evaluates the utility of the sediment quality triad when assessing the impact of agricultural activity on invertebrate growth, reproduction, and survival. In analyzing sediment and water chemistry, as well as metal and pesticide levels, none of the predictor variables were able to adequately explain the variation seen in any of the biological endpoints (reproduction, mortality, growth, or biomass). Although none of the factors measured in this experiment could explain the variation seen in biological endpoints, the experimental approach was informative in delineating biological trends between sediments subject to varying levels of agricultural activity. Although an experiment of this nature was not able to identify a causal mechanism to explain the variation in invertebrate biological endpoint, it is still extremely useful as an exploratory approach to assess relative sediment toxicity.





Similar content being viewed by others
References
Allan, J. D. (2004). Influence of land use and landscape setting on the ecological status of rivers. Limnetica, 23, 187–198. https://doi.org/10.1146/annurev.ecolsys.35.120202.110122.
Allan, J. D., Erickson, D. L., & Fay, J. (1997). The influence of catchment land use on stream integrity across multiple spatial scales. Freshwater Biolology, 37, 149–161. https://doi.org/10.1046/j.1365-2427.1997.d01-546.x.
Barbour, M. T., Gerritsen, J., Snyder, B. D., Stribling, J. B. (1999). USEPA rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish, Second edition. EPA 841-B-99-002. U.S. Environmental Protection Agency; Office of Water; Washington, D.C.
Beketov, M. (2004). Different sensitivity of mayflies (Insecta, Ephemeroptera) to ammonia, nitrite and nitrate: linkage between experimental and observational data. Hydrobiologia, 528, 209–216.
Borgmann, U., Ralph, K. M., & Norwood, W. P. (1989). Toxicity test procedures for Hyalella azteca, and chronic toxicity of cadmium and pentachlorophenol to H-azteca, Gammarus-fasciatus, and Daphnia magna. Archives of Environmental Contamination and Toxicology, 18, 756–764. https://doi.org/10.1007/BF01225013.
Burt, A. J., McKee, P. M., Hart, D. R., & Kauss, P. B. (1991). Effects of pollution on benthic invertebrate communities of the St. Marys River, 1985. Hydrobiologia, 219, 63–81. https://doi.org/10.1007/BF00024747.
Carpenter, S. R., Cole, J. J., Pace, M. L., Van de Bogert, M., Bade, D. L., Bastviken, D., Gille, C. M., Hodgson, J. R., Kitchell, J. F., & Kritzberg, E. S. (2005). Ecosystems subsidies: terrestrial support of aquatic food webs from 13C addition to contrasting lakes. Ecology, 86, 2737–2750.
Casellato, S. (1996). Oligochaetes in the southern basin of the Venetian Lagoon: community composition, species abundance and biomass. Hydrobiologia, 334, 103–114. https://doi.org/10.1007/BF00017359.
CCME. (2001). CCME protocol for the derivation of Canadian sediment quality guidelines for the protection of aquatic life. Transport, 1–4.
Chapman, P. M. (1990). The sediment quality triad approach to determining pollution-induced degradation. Science of the Total Environment, 97(98), 815–825. https://doi.org/10.1016/0048-9697(90)90277-2.
Compin, A., & Céréghino, R. (2003). Sensitivity of aquatic insect species richness to disturbance in the Adour-Garonne stream system (France). Ecological Indicators, 3, 135–142.
Cooper, C. M. (1993). Biological effects of agriculturally derived surface water pollutants on aquatic systems—a review. Journal of Environmental Quality, 22, 402. https://doi.org/10.2134/jeq1993.00472425002200030003x.
Courtney, L. A., & Clements, W. H. (1998). Effects of acidic pH on benthic macroinvertebrate communities in stream microcosms. Hydrobiologia, 379(1–3), 135–145.
Cuffney, T. F., Meador, M. R., Porter, S. D., & Gurtz, M. E. (2000). Responses of physical, chemical, and biological indicators of water quality to a gradient of agricultural land use in the Yakima river basin, Washington. Environmental Monitoring and Assessment, 64, 259–270. https://doi.org/10.1023/A:1006473106407.
Egler, M., Buss, D., Moreira, J., & Baptista, D. (2012). Influence of agricultural land-use and pesticides on benthic macroinvertebrate assemblages in an agricultural river basin in Southeast Brazil. Brazilian Journal of Biology, 72, 437–443. https://doi.org/10.1590/S1519-69842012000300004.
Fils Mamert, O., Serge Hubert, Z. T., Ernest, K., Nectaire Lie, N. T., & Simenon, T. (2016). Influence of municipal and industrial pollution on the diversity and the structure of benthic macro-invertebrates community of an urban stream in Douala, Cameroon. Journal of Biodiversity and Environmental Sciences, 8, 120–133.
Gan, J., Lee, S. J., Liu, W. P., Haver, D. L., & Kabashima, J. N. (2005). Distribution and persistence of pyrethroids in runoff sediments. Journal of Environmental Quality, 34, 836–841.
Giller, P. S. (2005). River restoration: seeking ecological standards. Editor’s introduction. Journal of Applied Ecology, 42, 201–207. https://doi.org/10.1111/j.1365-2664.2005.01020.x.
Gillis, P. L., Diener, L. C., Reynoldson, T. F., & Dixon, D. G. (2002). Cadmium-induced production of a metallothionein-like protein in Tubifex tubifex (Oligochaeta) and Chironomus riparius (Diptera): correlation with reproduction and growth. Environmental Toxicology and Chemistry, 21, 1836–1844.
Glass, G. V., Peckham, P. D., & Sanders, J. R. (1972). Consequences of failure to meet assumptions underlying fixed effects analyses of variance and covariance. Review of Educational Research, 42, 237–288.
Harwell, M. R., Rubinstein, E. N., Hayes, W. S., & Olds, C. C. (1992). Summarizing Monte Carlo results in methodological research: the one- and two-factor fixed effects ANOVA cases. Journal of Statistics Education, 17, 315–339.
Herman, M. R., & Najadhashemi, A. P. (2015). A review of macroinvertebrates- and fish-based stream health indices. Ecohydrology and Hydrobiology, 15, 53–67.
Herringshaw, C. J., Stewart, T. W., Thompson, J. R., & Anderson, P. F. (2011). Land use, stream habitat and benthic invertebrate assemblages in a highly altered Iowa watershed. The American Midland Naturalist, 165, 274–293. https://doi.org/10.1674/0003-0031-165.2.274.
Hurlbert, S. H. (1984). Pseudoreplication and the design of ecological field experiments. Ecological Monographs, 54(2), 187–211.
Hung, C., Gong, G., Chen, H., Hsieh, H., Santschi, P. H., Wade, T. L., & Sericano, J. S. (2007). Relationship between pesticide and organic carbon fractions in sediments of the Danshui River estruary and adjacent coastal areas of Taiwan. Environmental Pollution, 148, 546–554.
Jaagumagi, R., & Persaud, D. (1993). Sediment assessment: a guide to study design, sampling, and laboratory analysis. Toronto: Queen’s Printer for Ontario.
Johnston, J. M., Barber, M. C., Wolfe, K., Galvin, M., Cyterski, M., & Parmar, R. (2017). An integrated ecological modeling system for assessing impacts of multiple stressors on stream and riverine ecosystem services within river basin. Ecological Modelling, 354, 104–114.
Jones, C., Somers, K. M., Craig, B., & Reynoldson, T. B. (2007). Ontario Benthos Biomonitoring Network: protocol manual. Toronto: Queens Printer for Ontario.
Koop, J. H. E., Winkelmann, C., Becker, J., Hellmann, C., & Ortmann, C. (2011). Physiological indicators of fitness in benthic invertebrates; a useful measure for ecological health assessment and experimental biology. Aquatic Ecology, 45, 547–559.
Lantz, B. (2013). The impact of sample non-normality on ANOVA and alternative methods. The British Journal of Mathematical and Statistical Psychology, 66(2), 224–244.
Lenat, D. R., & Crawford, J. K. (1994). Effects of land use on water quality and aquatic biota of three North Carolina Piedmont streams. Hydrobiologia, 294, 185–199.
Lix, L. M., Keselman, J. C., & Keselman, H. J. (1996). Consequences of assumption violations revisited: a quantitative review of alternatives to the one-way analysis of variance F test. Review of Educational Research, 66, 579–619.
López-Doval, J. C., Großschartner, M., Höss, S., Orendt, C., Traunspurger, W., Wolfram, G., & Muñoz, I. (2010). Invertebrate communities in soft sediments along a pollution gradient in a Mediterranean river (Llobregat, NE Spain). Limnetica, 29, 311–322. https://doi.org/10.1111/1467-9906.00002.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31. https://doi.org/10.1007/s002440010075.
Matlou, K., Addo-Bediako, A., & Jooste, A. (2017). Benthic macroinvertebrate assemblage along a pollution gradient in the Steelpoort River, Olifants River system. African Entomology, 25, 445–453. https://doi.org/10.4001/003.025.0445.
Matthaei, C. D., Weller, F., Kelly, D. W., & Townsend, C. R. (2006). Impacts of fine sediment addition to tussock, pasture, dairy and deer farming streams in New Zealand. Freshwater Biology, 51, 2154–2172. https://doi.org/10.1111/j.1365-2427.2006.01643.x.
McTammany, M. E., Benfield, E. F., & Webster, J. R. (2007). Recovery of stream ecosystem metabolism from historical agriculture. Journal of the North American Benthological Society, 26, 532–545. https://doi.org/10.1899/06-092.1.
Meyer, J. L., Sale, M. J., Mulholland, P. J., & Poff, N. L. (1999). Impacts of climate change on aquatic ecosystem functioning and health. Journal of the American Water Resources Association, 35, 1373–1386. https://doi.org/10.1111/j.1752-1688.1999.tb04222.x.
Milani, D., Reynoldson, T. B., Borgmann, U., & Kolasa, J. (2003). The relative sensitivity of four benthic invertebrates to metals in spiked-sediment exposures and application to contaminated field sediment. Environmental Toxicology and Chemistry, 22, 845–854. https://doi.org/10.1002/etc.5620220424.
MOECC. (2012). Standard operating procedure: Hexagenia spp. culturing. SOP HX1.v5. Ontario Ministry of the Environment; Laboratory Services Branch; Aquatic Toxicology Unit; Toronto, Ontario, Canada.
Moore, A. A., & Palmer, M. A. (2005). Invertebrate biodiversity in agricultural and urban headwater streams: implications for conservation and management. Ecological Applications, 15, 1169–1177. https://doi.org/10.1890/04-1484.
Nakano, S., & Murakami, M. (2001). Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proceedings of the National Academy of Sciences of the United States of America, 98, 166–170.
Nilsson, C., & Berggren, K. (2000). Alterations of riparian ecosystems caused by river regulation. Bioscience, 50, 783–792. https://doi.org/10.1641/0006-3568(2000)050[0783:AORECB]2.0.CO;2.
OMAFRA. 2016. Area of census farm (acres) by county, Ontario: 2016. http://www.omafra.gov.on.ca/english/stats/census/cty30a.htm
Ometo, J. P. H. B., Martinelli, L. A., Ballester, M. V., Gessner, A., Krusche, A. V., Victoria, R. L., & Williams, M. (2000). Effects of land use on water chemistry and macroinvertebrates in two streams of the Piracicaba river basin, south-east Brazil. Freshwater Biology, 44, 327–337. https://doi.org/10.1046/j.1365-2427.2000.00557.x.
OMNRF (Ontario Ministry of Natural Resources and Forestry). (2017). User guide for Ontario Flow Assessment Tool (OFAT). Provincial Mapping Unit, Mapping and Information Resources Branch.
Pace, M. L., Cole, J. J., Carpenter, S. R., Kitchell, J. F., Hodgson, J. R., Van de Bogert, M. C., Bade, D. L., Kritzberg, E. S., & Bastviken, D. (2004). Whole-lake carbon-13 additions reveal terrestrial support of aquatic food webs. Nature, 427, 240–243.
Pavlin, M., Birk, S., Hering, D., & Urbanič, G. (2011). The role of land use, nutrients, and other stressors in shaping benthic invertebrate assemblages in Slovenian rivers. Hydrobiologia, 678, 137–153. https://doi.org/10.1007/s10750-011-0836-8.
Prosser, R. S., Bartlett, A. J., Milani, D., Holman, E. A. M., Ikert, H., Schissler, D., Toito, J., Parrott, J. L., Gillis, P. L., & Balakrishnan, V. K. (2017a). Variation in the toxicity of sediment-associated substituted phenylamine antioxidants to an epibenthic (Hyalella azteca) and endobenthic (Tubifex tubifex) invertebrate. Chemosphere, 181, 250–258. https://doi.org/10.1016/j.chemosphere.2017.04.066.
Prosser, R. S., Parrott, J. L., Galicia, M., Shires, K., Sullivan, C., Toito, J., Bartlett, A. J., Milani, D., Gillis, P. L., & Balakrishnan, V. K. (2017b). Toxicity of sediment-associated substituted phenylamine antioxidants on the early life stages of Pimephales promelas and a characterization of effects on freshwater organisms. Environmental Toxicology and Chemistry, 36, 2730–2738.
Reynoldson, T. B. (1994). A field test of a sediment bioassay with the oligochaete worm Tubifex tubifex (Müller, 1774). Hydrobiologia, 278, 223–230.
Reynoldson, T. B., & Metcalfe-Smith, J. L. (1992). An overview of the assessment of aquatic ecosystem health using benthic invertebrates. Aquatic Ecosystem Health, 1, 295–308.
Reynoldson, T. B., Thompson, S. P., & Bamsey, J. L. (1991). A sediment bioassay using the tubificid oligochaete worm Tubifex tubifex. Environmental Toxicology and Chemistry, 10, 1061–1072.
Richards, C., Host, G. E., & Arthur, J. W. (1993). Identification of predominant environmental factors structuring stream macroinvertebrate communities within a large agricultural catchment. Freshwater Biology, 29, 285–294. https://doi.org/10.1111/j.1365-2427.1993.tb00764.x.
Rubach, M. N., Ashauer, R., Buchwalter, D. B., De Lange, H. J., Hamer, M., Preuss, T. G., Töpke, K., & Maund, S. J. (2011). Framework for traits-based assessment in ecotoxicology. Integrated Environmental Assessment and Management, 7, 172–186. https://doi.org/10.1002/ieam.105.
Scott, M. C., & Helfman, G. S. (2001). Native invasions, homogenization, and the mismeasure of integrity of fish assemblages. Fisheries, 26, 6–15. https://doi.org/10.1577/1548-8446(2001)026<0006:NIHATM>2.0.CO;2.
Smith, S. L., MacDonald, D. D., Keenleyside, K. A., Ingersoll, C. G., & Field, L. J. (1996). A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. Journal of Great Lakes Research, 22, 624–638. https://doi.org/10.1016/S0380-1330(96)70985-1.
Søndergaard, M., & Jeppesen, E. (2007). Anthropogenic impacts on lake and stream ecosystems, and approaches to restoration. Journal of Applied Ecology, 44, 1089–1094. https://doi.org/10.1111/j.1365-2664.2007.01426.x.
Stepenuck, K. F., Crunkilton, R. L., & Wang, Z. (2002). Impacts of urban landuse on macroinvertebrate communities in southeastern Wisconsin streams. Journal of American Water Resources Association, 38, 1041–1051.
Storey, R. G., & Cowley, D. R. (1997). Recovery of three New Zealand rural streams as they pass through native forest remnants. Hydrobiologia, 353, 63–76. https://doi.org/10.1023/A:1003042425431.
Strayer, D. L., Beighley, R. E., Thompson, L. C., Brooks, S., Nilsson, C., Pinay, G., & Naiman, R. J. (2003). Effects of land cover on stream ecosystems: roles of empirical models and scaling issues. Ecosystems, 6, 407–423. https://doi.org/10.1007/s10021-002-0170-0.
Suedel, C. B., & Rodgers Jr., J. J. (1994). Responses of Hyalella Azteca and Chironomus Tentans to particle-size distribution and organic matter content of formulated and natural freshwater sediments. Environmental Toxicology and Chemistry, 13, 1639–1648. https://doi.org/10.1002/etc.5620131013.
Tang, H., Song, M.-Y., Cho, W.-S., Park, Y.-S., & Chon, T.-S. (2010). Species abundance distribution of benthic chironomids and other macroinvertebrates across different levels of pollution in streams. Annales de Limnologie—Internal Journal of Limnology, 46, 53–66. https://doi.org/10.1051/limn/2009031.
Townsend, C. R., Dolédec, S., Norris, R., Peacock, K., & Arbuckle, C. (2003). The influence of scale and geography on relationships between stream community composition and landscape variables: description and prediction. Freshwater Biology, 48, 768–785. https://doi.org/10.1046/j.1365-2427.2003.01043.x.
Virbickas, T., Pliuraite, V., & Kesminas, V. (2011). Impact of agricultural land use on macroinvertebrate fauna in Lithuania. Polish Journal of Environmental Studies, 20, 1327–1334.
Wang, L., Lyons, J., Kanehl, P., & Gatti, R. (1997). Influences of watershed land use on habitat quality and biotic integrity in Wisconsin streams. Fisheries Magazine, 22, 6–12.
Wood, P. J., & Armitage, P. D. (1997). Biological effects of fine sediment in the lotic environment. Environmental Management, 21, 203–217. https://doi.org/10.1002/hyp.7604.
Wright, L. L., & Mattice, J. S. (1981). Substrate selection as a factor in Hexagenia distribution. Aquatic Insects, 3, 13–24. https://doi.org/10.1080/01650428109361039.
Wright, I. A., & Ryan, M. M. (2016). Impact of mining and industrial pollution on stream macroinvertebrates: importance of taxonomic resolution, water geochemistry and EPT indices for impact detection. Hydrobiologia, 772, 103–115. https://doi.org/10.1007/s10750-016-2644-7.
Yuan, L. (2010). Estimating the effects of excess nutrients on invertebrates from observational data. Ecological Applications, 20(1), 110–125.
Acknowledgments
The authors would like to thank the Long Point Region Conservation Authority, the Kettle Creek Conservation Authority, the farmers who allowed us access to their land, and Emelia Myles-Gonzalez, Laura Johnson, Kathleen Lo, and Eric Johnson for their time and contributions to the study.
Funding
This work was supported by the Canada First Research Excellence Fund – Food For Thought Grant to K.S. McCann and Natural Science and Engineering Research Council Discovery Grant to R.S. Prosser. The funding source had no such involvement in the study design, collection, analysis, or interpretation of the data. There was also no involvement in the writing of the report or the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 1.91 mb)
Rights and permissions
About this article
Cite this article
Wolf, J.F., Prosser, R.S., Champagne, E.J. et al. Variation in Response of Laboratory-Cultured Freshwater Macroinvertebrates to Sediment from Streams with Differential Exposure to Agriculture. Water Air Soil Pollut 231, 13 (2020). https://doi.org/10.1007/s11270-019-4376-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4376-6