Abstract
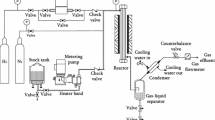
In this study, 27 groups of hydrogenation experiments were carried out in a single-tube fixed-bed reactor using 9 phenolic compounds extracted from coal tar (fraction before 240 °C) as raw materials. Based on the experimental results, a set of molecular reaction kinetics model which involve 21 compounds and 33 chemical reactions was established. In this paper, the Runge–Kutta method was used to solve the dynamic equations, and the BFGS algorithm (a quasi-Newton optimization algorithm) is used to optimize the parameters of the dynamic model. The whole calculation process was completed on MATLAB software. The experimental results show that the maximum error of the model for predicting phenolic, unsaturated hydrocarbon and saturated hydrocarbon content in products is less than 5%, while the maximum error for predicting single substance is less than 10%. It could be calculated that the optimum temperature for hydrodeoxygenation of phenolic compounds is 633 K, which aims to retain aromatic hydrocarbons.







Similar content being viewed by others
References
Li D, Li Z, Li W, Liu Q, Feng Z, Fan Z (2013) Hydrotreating of low temperature coal tar to produce clean liquid fuels. Anal Appl Pyrolysis 100:245–252
Li C, Suzuki K (2010) Resources, properties and utilization of tar. Resour Conserv Recycl 54:905–915
Sun M, Ma X, Yao Q, Wang R, Ma Y, Feng G, Shang J, Xu L, Yang Y (2011) GC-MS and TG-FTIR study of petroleum ether extract and residue from low temperature coal tar. Energy Fuels 25:1140–1145
Niu M, Sun X, Li D, Cun W, Bai X, Zhang X, Li W (2017) The hydrodeoxygenation, hydrogenation, hydrodealkylation and ring-opening reaction in the hydrotreating of low temperature coal tar over Ni–Mo/γ-Al2O3 catalyst. Reac Kinet Mech Cat 121:1–17
Kan T, Wang HY, He HX, Li CS, Zhang SJ (2011) Experimental study on two-stage catalytic hydroprocessing of middle-temperature coal tar to clean liquid fuels. Fuel 90:3404–3409
Bollas GM, Lappas AA, Iatridis DK, Vasalos IA (2007) Five-lump kinetic model with selective catalyst deactivation for the prediction of the product selectivity in the fluid catalytic cracking process. Catal Today 127:31–43
Meng X, Xu C, Gao J, Li L (2006) Catalytic pyrolysis of heavy oils: 8-lump kinetic model. Appl Catal A 301:32–38
Ng S, Wang J, Zhu Y, Zheng L, Ding F, Yang L, Yui S (2002) A new approach to determining product selectivity in gas oil cracking using a four-lump kinetic model. Energy Fuels 16:593–600
Dave NC, Duffy GJ, Udaja P (1993) A four-lump kinetic model for the cracking/coking of recycled heavy oil. Fuel 72:1331–1334
Zhu YH, Zhang YH, Dan Y, Yuan Y, Zhang L, Li WH, Li D (2015) Optimization of reaction variables and macrokinetics for the hydrodeoxygenation of full range low temperature coal tar. Reac Kinet Mech Cat 116:433–450
Sun J, Li D, Yao R, Sun Z, Li X, Li W (2015) Modeling the hydrotreatment of full range medium temperature coal tar by using lumping kinetic approach. Reac Kinet Mech Cat 114:451–471
Niu M, Zheng H, Sun X, Zhang S, Li D, Qiao J, Li W (2017) Kinetic model for low-temperature coal tar hydrorefining. Energy Fuels 31:5441–5447
Valencia D, Klimova T (2012) Kinetic study of NiMo/SBA-15 catalysts prepared with citric acid in hydrodesulfurization of dibenzothiophene. Catal Commun 21:77–81
Kallinikos LE, Jess A, Papayannakos NG (2010) Kinetic study and H2S effect on refractory DBTs desulfurization in a heavy gas oil. J Catal 269:169–178
Castillo-Araiza CO, Chávez G, Dutta A, Reyes JADL, Nuñez S, García-Martínez JC (2015) Role of Pt–Pd/γ-Al2O3, on the HDS of 4,6-DMBT: kinetic modeling & contribution analysis. Fuel Process Technol 132:164–172
Niu ML, Sun XH, Gao R, Li D, Cui WG, Li WH (2016) Effect of dephenolization on low-temperature coal tar hydrogenation to produce fuel oil. Energy Fuels 30:10215–10221
Wandas R, Surygala J, Śliwka E (1996) Conversion of cresols and naphthalene in the hydroprocessing of three-component model mixtures simulating fast pyrolysis tars. Fuel 75:687–694
Messenger L, Attar A (1979) Thermodynamics of the transformations of oxygen-and sulphur-containing functional groups during coal liquefaction in hydrogen and hydrogen donor. Fuel 58:655–660
Romero Y, Richard F, Brunet S (2010) Hydrodeoxygenation of 2-ethylphenol as a model compound of bio-crude over sulfided Mo-based catalysts: promoting effect and reaction mechanism. Appl Catal B 98(3):213–223
Gevert SB, Eriksson M, Eriksson P (1994) Direct hydrodeoxygenation and hydrogenation of 2,6- and 3,5-dimethylphenol over sulphided CoMo catalyst. Appl Catal A 117(2):151–162
Souza PMD, Lei N, Borges LEP (2014) Role of oxophilic supports in the selective hydrodeoxygenation of m-cresol on Pd catalysts. Catal Lett 144(12):2005–2011
Ramage MP, Graziani KR, Krambeck FJ (1980) Development of mobils kinetic reforming model. Chem Eng Sci 35:41–48
Froment GF (1987) The kinetic of complex catalytic reactions. Chem Eng Sci 42:1073–1087
Jenkins JH, Stephens TW (1980) Kinetics of catalytic reforming. Hydrocarb Process 59:163–167
Levenspiel O (1972) Experimental search for a simple rate equation to describe deactivating porous catalyst particles. J Cata 25:265–272
Hong Y, Wang Y (2017) Elucidation of reaction mechanism for, m-cresol hydrodeoxygenation over Fe based catalysts: a kinetic study. Catal Commun 100:43–47
Hachemi I, Murzin DY (2017) Kinetic modeling of fatty acid methyl esters and triglycerides hydrodeoxygenation over nickel and palladium catalysts. Chem Eng J 334:2201–2207
Qadar SA, Wiser WH, Hill GR (1968) Kinetics of hydrocracking of low temperature coal tar. Am. Chem. Soc. Div. Fuel Chem. Prepr 12:28–46
Gonc¸alves VOO, De Souza PM, Da Silva VT (2017) Kinetics of the hydrodeoxygenation of cresol isomers over Ni2P/SiO2: proposals of nature of deoxygenation active sites based on an experimental study. Appl Catal B 205:357–367
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, L., Niu, M., He, Y. et al. Molecular reaction kinetics model for the hydrodeoxygenation of low boiling point phenolic compounds in coal tar with Ni–Ce/SiO2 catalysts. Reac Kinet Mech Cat 128, 315–331 (2019). https://doi.org/10.1007/s11144-019-01612-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-019-01612-x