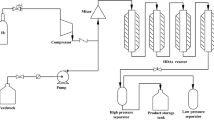
Abstract
The effects of operating conditions on the hydrodeoxygenation (HDO) of full-range low temperature coal tar (LCT) were investigated in a trickle-bed hydrogenation unit. The optimized process conditions analyzed by response surface methodology were found as: reaction temperature of 658.15 K, reaction pressure of 13.51 MPa, liquid hourly space velocity (LHSV) of 0.30 h−1, hydrogen to oil volume ratio of 1100 NL/L. The order of the importance on HDO was: LHSV > temperature > pressure. A new kinetic model for HDO of LCT has been also built based on the power law model. The estimation of model parameter was carried out by using Levenberg–Marquardt method. The results were validated by a series of further experiments. Comparisons between the experimental and predicted data showed good agreement. The relative error and absolute error from the combination kinetic model are both lower than 1.5 %.







Similar content being viewed by others
Abbreviations
- y HDO :
-
The hydrodeoxygenation conversion rate of coal tar
- LHSV:
-
Liquid hourly space velocity, h−1
- P :
-
Hydrogen partial pressure, MPa
- T :
-
Reaction temperature, K
- k :
-
Apparent reaction rate constant, (ppm)1−n ha MPa−b
- w :
-
Content of phenols in oil, ppm
- t :
-
Residence time, h
- n :
-
Reaction order
- w f :
-
Content of phenols in feed, ppm
- w p :
-
Content of phenols in product, ppm
- a :
-
LHSV index
- b :
-
Hydrogen pressure index
- k 0 :
-
M, Pre-exponential factor of Arrhenius equation, (ppm)1−n ha MPa−b
- E a :
-
Apparent activation energy, J mol−1
- R :
-
Gas constant, 8.314 J/(mol K)
References
Liu ZY, Shi SD, Li YW (2010) Coal liquefaction technologies-development in China and challenges in chemical reaction engineering. Chem Eng Sci 65:12–17
Nolan P, Shipman A, Rui H (2004) Coal liquefaction, Shenhua group, and China’s energy security. Eur Manag J 22:150–164
Sun JM, Li D, Yao RQ, Sun ZH, Li XK, Li WH (2014) Modeling the hydrotreatment of full range medium temperature coal tar by using a lumping kinetic approach. Reac Kinet Mech Cat 2:451–471
Li D, Li Z, Li WH, Liu QC, Feng ZL, Fan Z (2013) Hydrotreating of low temperature coal tar to produce clean liquid fuels. J Anal Appl Pyrolysis 100:245–252
Šafářová M, Kusý J, Anděl L (2010) Brown coal tar hydrotreatment. J Anal Appl Pyrolysis 89:265–270
Kan T, Wang HY, He HX, Li CS, Zhang SJ (2011) Experimental study on two-stage catalytic hydroprocessing of middle-temperature coal tar to clean liquid fuels. Fuel 90:3404–3409
Dai F, Gao MJ, Li CS, Xiang SG, Zhang SJ (2011) Detailed description of coal tar hydrogenation process using the kinetic lumping approach. Energy Fuel 25:4878–4885
Kan T, Sun XY, Wang HY, Li CS, Muhammad U (2012) Production of gasoline and diesel from coal tar via its catalytic hydrogenation in serial fixed beds. Energy Fuel 26:3604–3611
Kusy J, Andel L, Safarova M, Vales J, Ciahotny K (2012) Hydrogenation process of the tar obtained from the pyrolisis of brown coal. Fuel 101:38–44
Tang W, Fang MX, Wang HY, Yu PL, Wang QH, Luo ZY (2014) Mild hydrotreatment of low temperature coal tar distillate: product composition. Chem Eng J 236:529–537
Furimsky E (2000) Catalytic hydrodeoxygenation. Appl Catal Gen 199:147–190
Allen DT, Grandy DW, Jeong KM, Petrakis L (1985) Heavier fractions of shale oils, heavy crudes, tar sands, and coal liquids: comparison of structural profiles. Ind Eng Chem Process Des Dev 24:737–742
Burgess CE, Schobert HH (1998) Relationship of coal characteristics determined by pyrolysis/gas chromatography/mass spectrometry and nuclear magnetic resonance to liquefaction reactivity and product composition. Energy Fuel 12:1212–1222
Edwards JH, Schluter K, Tyler RJ (1986) Upgrading of flash pyrolysis tars to synthetic crude oil: 2. second-stage hydrotreatment using nickel/molybdenum catalysts. Fuel 65:202–207
Edwards JH, Schluter K, Tyler RJ (1986) Upgrading of flash pyrolysis tars to synthetic crude oil: 3. overall performance of the two-stage hydrotreating process and characterization of the synthetic crude oil. Fuel 65:208–211
Girgis MJ, Gates BC (1991) Reactivities, reaction networks, and kinetics in high-pressure catalytic hydroprocessing. Ind Eng Chem Res 30:2021–2058
Perot G (1991) The reactions involved in hydrodenitrogenation. Catal Today 10:447–472
Šaban MD, Skala DU, Jovanović JA (1992) Hydrodenitrogenation of aleksinac shale oil distillates in a pilot trickle-bed reactor. Fuel Process Technol 30:15–31
Diaz-Real RA, Mann RS, Sambi IS (1993) Hydrotreatment of Athabasca bitumen derived gas oil over nickel-molybdenum, nickel-tungsten, and cobalt-molybdenum catalysts. Ind Eng Chem Res 32:1354–1358
Kwak S, Longstaff DC, Deo MD (1994) Hydrotreatment process kinetics for bitumen and bitumen-derived liquids. Fuel 73:1531–1536
Yui SM, Ng SH (1995) Hydrotreating of a bitumen-derived coker HGO and evaluation of hydrotreated HGOs as potential FCC feeds using microactivity test unit. Energy Fuel 9:665–672
Kim JW, Longstaff DC, Hanson FV (1998) Catalytic and thermal effects during hydrotreating of bitumen-derived heavy oils. Fuel 77:1815–1823
Massoth FE, Politzer P, Concha MC, Murray JS, Jakowski J (2006) Catalytic hydrodeoxygenation of methyl-substituted phenols: correlations of kinetic parameters with molecular properties. J Phys Chem B 110:14283–14291
Laurent E, Delmon B (1993) Influence of oxygen-, nitrogen-, and sulfur-containing compounds on the hydrodeoxygenation of phenols over sulfided CoMo/γ-Al2O3 catalysts. Ind Eng Chem Res 32:2516–2524
Gevert SB, Eriksson M, Eriksson P (1994) Direct hydrodeoxygenation and hydrogenation of 2, 6-and 3, 5-dimethylphenol over sulphided CoMo catalyst. Appl Catal Gen 117:151–162
Lee CL, Ollis DF (1984) Catalytic hydrodeoxygenation of benzofuran and o-ethylphenol. J Catal 87:325–331
Satterfield CN, Yang SH (1983) Simultaneous hydrodenitrogenation and hydrodeoxygenation of model compounds in a trickle bed reactor. J Catal 81:335–346
Girgis MJ, Gates BC (1994) Catalytic hydroprocessing of simulated heavy coal liquids. 1. reactivities of aromatic hydrocarbons and sulfur and oxygen heterocyclic compounds. Ind Eng Chem Res 33:1098–1106
Artok L, Erbatur O, Schobert HH (1996) Reaction of dinaphthyl and diphenyl ethers at liquefaction conditions. Fuel Process Technol 47:153–176
Furimsky E, Ranganathan R, Parsons BI (1978) Catalytic hydrodenitrogenation of basic and non-basic nitrogen compounds in Athabasca bitumen distillates. Fuel 57:427–430
Petrakis L, Ruberto RG, Young DC (1983) Catalytic hydroprocessing of SRC-II heavy distillate fractions. 1. Preparation of the fractions by liquid chromatography. Ind Eng Chem Process Des Dev 22:292–298
Katti SS, Westerman DWB, Gates BC (1984) Catalytic hydroprocessing of SRC-II heavy distillate fractions. 3. Hydrodesulfurization of the neutral oils. Ind Eng Chem Process Des Dev 23:773–778
Grandy DW, Petrakis L, Li CL (1986) Catalytic hydroprocessing of SRC-II heavy distillate fractions. 5. Conversion of the acidic fractions characterized by gas chromatography/mass spectrometry. Ind Eng Chem Process Des Dev 25:40–48
Li CL, Xu ZR, Gates BC (1985) Catalytic hydroprocessing of SRC-II heavy distillate fractions. 4. Hydrodeoxygenation of phenolic compounds in the acidic fractions. Ind Eng Chem Process Des Dev 24:92–97
Chon S, Allen DT (1991) Catalytic hydroprocessing of chlorophenols. AIChE J 37:1730–1732
Wang WY, Yang YQ, Luo H, Liu WY (2010) Effect of Co on Ni–Mo–B amorphous catalyst in hydrodeoxygenation. CIESC J 61:73–79
Viljava TR, Komulainen RS, Krause AOI (2000) Effect of H2S on the stability of CoMo/Al2O3 catalysts during hydrodeoxygenation. Catal Today 60:83–92
Yang YQ, Luo HA, Tong GS (2008) Hydrodeoxygenation of phenolic model compounds over MoS2 catalysts with different structures. Chin J Chem Eng 16:733–739
Sato Y (1997) Hydrotreating of heavy distillate derived from Wandoan coal liquefaction. Catal Today 39:89–98
Leckel D (2006) Catalytic hydroprocessing of coal-derived gasification residues to fuel blending stocks: effect of reaction variables and catalyst on hydrodeoxygenation (HDO), hydrodenitrogenation (HDN), and hydrodesulfurization (HDS). Energy Fuel 20:1761–1766
Sun ZH, Li D, Li WH, Li Z, Li YC (2013) Kinetics of coal tar hydrodenitrogenation. Acta Pet Sinica 29:1035–1039
Ternan M, Brown JR (1982) Hydrotreating a distillate liquid derived from subbituminous coal using a sulphided CoO–MoO3–Al2O3 catalyst. Fuel 61:1110–1118
Leckel D (2007) Hydrodeoxygenation of heavy oils derived from low-temperature coal gasification over NiW catalysts-effect of pore structure. Energy Fuel 22:231–236
White PJ, Jones JF, Eddinger RT (1968) To treat and crack oil from coal. Hydrocarb Process A 47:97–99
Krishnamurthy S, Panvelker S, Shah YT (1981) Hydrodeoxygenation of dibenzofuran and related compounds. AIChE J 27:994–1001
Su-Ping Z (2003) Study of hydrodeoxygenation of bio-oil from the fast pyrolysis of biomass. Energy Sources 25:57–65
Acknowledgments
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (21206136), Overall Science and Technology Innovation Project of Shaanxi province (2014KTCL01-09) and Research Fund for the Doctoral Program of Higher Education of China (20126101120013) and Scientific Research Project of the Department of Education of Shaanxi province (2013JK0692, 2013JC21).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Y., Zhang, Y., Dan, Y. et al. Optimization of reaction variables and macrokinetics for the hydrodeoxygenation of full range low temperature coal tar. Reac Kinet Mech Cat 116, 433–450 (2015). https://doi.org/10.1007/s11144-015-0900-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-015-0900-x