Abstract
Background and aims
Our recent research revealed that several key enzymes involved in flavonoid biosynthesis played vital roles in soybean’s tolerance to salinity. Since the flavonoids also act as important signals mediating the establishment of symbiosis between rhizobium and leguminous plants, the Sinorhizobium meliloti 1021 was tested to find out whether it had any impact on soybean’s tolerance to salt stress.
Methods
The roots of soybean seedling (Glycine max cultivar Union85140) were inoculated by soak in diluted S. meliloti 1021 suspension and then the seedlings were transferred in sphagnum peat and pearlite soil. Five days after inoculation, the seedlings were treated with salt solutions every four days until harvest.
Results
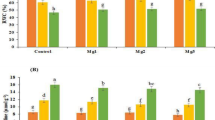
Soybean seedlings inoculated with S. meliloti 1021 demonstrated significantly stronger lodging-resistance and the fresh weight of the whole plants increased by 10.95–30.95 % compared with that of the non-inoculated plants. These changes in soybean seedlings might be resulted from: 1. S. meliloti 1021 playing a role in helping soybean plant to exclude Na+ while absorb K+, reducing ionic stress caused by salts; 2. S. meliloti 1021 enhancing the antioxidant enzymes' activities on reducing oxidative stress caused by salts; 3. S. meliloti 1021 increasing the content of osmotic compounds, thus reducing osmotic stress caused by salts. In addition, S. meliloti 1021 played significant roles in regulating the transcription of several key enzymes related to flavonoids metabolism (cytochrome P450 monooxygenase, chalcone synthase and chalcone isomerase), ROS scavenging (catalases, ascorbate peroxidase, glutathione S-transferase and superoxide dismutase), and other salt-responsive genes (Stress-induced protein SAM22, PR10-like protein and Phosphatidylinositol-specific phospholipase C) in soybean seedlings.
Conclusion
These results showed that S. meliloti 1021 significantly enhanced soybean’s ability to adapt to saline soil, mostly due to its effect on flavonoids metabolism in plants.









Similar content being viewed by others
References
Affenzeller MJ, Darehshouri A, Andosch A, Lutz C, Lutz-Meindl U (2009) Salt stress-induced cell death in the unicellular green alga micrasterias denticulata. J Exp Bot 60:939–954. doi:10.1093/Jxb/Ern348
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interations with plants and other organisms. Annu Rev Plant Biol 57:233–266. doi:10.1146/annurev.arplant.57.032905.105159
Belcarz A, Ginalska G, Kowalewska B, Kulesza P (2008) Spring cabbage peroxidases-potential tool in biocatalysis and bioelectrocatalysis. Phytochemistry 69:627–636. doi:10.1016/j.phytochem.2007.08.018
Bianco C, Defez R (2009) Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J Exp Bot 60:3097–3107. doi:10.1093/jxb/erp140
Chavez-Aviles MN, Andrade-Perez CL, de la Cruz HR (2013) PP2A mediates lateral root development under NaCl-induced osmotic stress throughout auxin redistribution in Arabidopsis thaliana. Plant Soil 368:591–602. doi:10.1007/s11104-012-1540-9
Chen SL, Hawighorst P, Sun J, Polle A (2014a) Salt tolerance in populus: significance of stress signaling networks, mycorrhization, and soil amendments for cellular and whole-plant nutrition. Environ Exp Bot 107:113–124. doi:10.1016/j.envexpbot.2014.06.001
Chen Y, Lin FZ, Yang H, Yue L, Hu F, Wang JL, Luo YY, Cao FL (2014b) Effect of varying NaCl doses on flavonoid production in suspension cells of Ginkgo biloba: relationship to chlorophyll fluorescence, ion homeostasis, antioxidant system and ultrastructure. Acta Physiol Plant 36:3173–3187. doi:10.1007/s11738-014-1684-8
Coberly L, Rausher M (2003) Analysis of a chalcone synthase mutant in ipomoea purpurea reveals a novel function for flavonoids: amelioration of heat stress. Mol Ecol 12:1113–1124
Complainville A, Brocard L, Roberts I, Dax E, Sever N, Sauer N, Kondorosi A, Wolf S, Oparka K, Crespi M (2003) Nodule initiation involves the creation of a new symplasmic field in specific root cells of medicago species. Plant Cell 15:2778–2791. doi:10.1105/tpc.017020
Dardanelli MS, Manyani H, Gonzalez-Barroso S, Rodriguez-Carvajal MA, Gil-Serrano AM, Espuny MR, Lopez-Baena FJ, Bellogin RA, Megias M, Ollero FJ (2010) Effect of the presence of the plant growth promoting rhizobacterium (PGPR) chryseobacterium balustinum Aur9 and salt stress in the pattern of flavonoids exuded by soybean roots. Plant Soil 328:483–493. doi:10.1007/s11104-009-0127-6
De Carvalho-Niebel F, Timmers AC, Chabaud M, Defaux-Petras A, Barker DG (2002) The nod factor-elicited annexin MtAnn1 is preferentially localised at the nuclear periphery in symbiotically activated root tissues of Medicago truncatula. Plant J 32:343–352
Dudonne S, Vitrac X, Coutiere P, Woillez M, Merillon JM (2009) Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem 57:1768–1774. doi:10.1021/Jf803011r
Echeverria M, Sannazzaro AI, Ruiz OA, Menendez AB (2013) Modulatory effects of mesorhizobium tianshanense and Glomus intraradices on plant proline and polyamine levels during early plant response of lotus tenuis to salinity. Plant Soil 364:69–79. doi:10.1007/s11104-012-1312-6
Egamberdieva D, Berg G, Lindstrom K, Rasanen LA (2013) Alleviation of salt stress of symbiotic galega officinalis L. (goat’s rue) by co-inoculation of rhizobium with root-colonizing pseudomonas. Plant Soil 369:453–465. doi:10.1007/s11104-013-1586-3
Elmajdoub B, Barnett S, Marschner P (2014) Response of microbial activity and biomass in rhizosphere and bulk soils to increasing salinity. Plant Soil 381:297–306. doi:10.1007/s11104-014-2127-4
Estrada B, Barea JM, Aroca R, Ruiz-Lozano JM (2013) A native Glomus intraradices strain from a mediterranean saline area exhibits salt tolerance and enhanced symbiotic efficiency with maize plants under salt stress conditions. Plant Soil 366:333–349. doi:10.1007/s11104-012-1409-y
Gleason C, Chaudhuri S, Yang T, Munoz A, Poovaiah BW, Oldroyd GE (2006) Nodulation independent of rhizobia induced by a calcium-activated kinase lacking autoinhibition. Nature 441:1149–1152. doi:10.1038/nature04812
Gong XL, Chao L, Zhou M, Hong MM, Luo LY, Wang L, Ying W, Cai JW, Gong SJ, Hong FS (2011) Oxidative damages of maize seedlings caused by exposure to a combination of potassium deficiency and salt stress. Plant Soil 340:443–452. doi:10.1007/s11104-010-0616-7
Gutjahr C, Banba M, Croset V, An K, Miyao A, An G, Hirochika H, Imaizumi-Anraku H, Paszkowski U (2008) Arbuscular mycorrhiza-specific signaling in rice transcends the common symbiosis signaling pathway. Plant Cell 20:2989–3005. doi:10.1105/tpc.108.062414
Gyorgypal Z, Kondorosi A (1991) Homology of the ligand-binding regions of rhizobium symbiotic regulatory protein NodD and vertebrate nuclear receptors. Mol Gen Genet 226:337–340
Hajiboland R, Aliasgharzadeh N, Laiegh SF, Poschenrieder C (2010) Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 331:313–327. doi:10.1007/s11104-009-0255-z
Hessini K, Ben Hamed K, Gandour M, Mejri M, Abdelly C, Cruz C (2013) Ammonium nutrition in the halophyte Spartina alterniflora under salt stress: evidence for a priming effect of ammonium? Plant Soil 370:163–173. doi:10.1007/s11104-013-1616-1
Kanawapee N, Sanitchon J, Srihaban P, Theerakulpisut P (2013) Physiological changes during development of rice (Oryza sativa L.) varieties differing in salt tolerance under saline field condition. Plant Soil 370:89–101. doi:10.1007/s11104-013-1620-5
Kopittke PM (2012) Interactions between Ca, Mg, Na and K: alleviation of toxicity in saline solutions. Plant Soil 352:353–362. doi:10.1007/s11104-011-1001-x
Lam HM, Xu X, Liu X, Chen WB, Yang GH, Wong FL, Li MW, He WM, Qin N, Wang B. Li J, Jian M, Wang J, Shao G, Wang J, Sun SS, Zhang G. (2010) Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat Genet 42: 1053-U1041. doi:10.1038/ng.715
Liu W, Kohlen W, Lillo A, Op den Camp R, Ivanov S, Hartog M, Limpens E, Jamil M, Smaczniak C, Kaufmann K, Yang WC, Hooiveld GJ, Charnikhova T, Bouwmeester HJ, Bisseling T, Geurts R (2011) Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell 23:3853–3865. doi:10.1105/tpc.111.089771
Lopez-Gomez M, Hidalgo-Castellanos J, Iribarne C, Lluch C (2014) Proline accumulation has prevalence over polyamines in nodules of Medicago sativa in symbiosis with Sinorhizobium meliloti during the initial response to salinity. Plant Soil 374:149–159. doi:10.1007/s11104-013-1871-1
Lu YH, Lam HM, Pi EX, Zhan QL, Tsai SN, Wang CM, Kwan YW, Ngai SM (2013) Comparative metabolomics in Glycine max and glycine soja under salt stress to reveal the phenotypes of their offspring. J Agric Food Chem 61:8711–8721. doi:10.1021/jf402043m
Marti MC, Florez-Sarasa I, Camejo D, Ribas-Carbo M, Lazaro JJ, Sevilla F, Jimenez A (2011) Response of mitochondrial thioredoxin PsTrxo1, antioxidant enzymes, and respiration to salinity in pea (pisum sativum L.) leaves. J Exp Bot 62:3863–3874. doi:10.1093/Jxb/Err076
Miransari M, Smith DL (2009) Alleviating salt stress on soybean (Glycine max (L.) merr.) - Bradyrhizobium japonicum symbiosis, using signal molecule genistein. Eur J Soil Biol 45:146–152. doi:10.1016/j.ejsobi.2008.11.002
Mitra RM, Gleason CA, Edwards A, Hadfield J, Downie JA, Oldroyd GE, Long SR (2004) A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: gene identification by transcript-based cloning. PNAS 101:4701–4705. doi:10.1073/pnas.0400595101
Pi E, Qu L, Hu J, Huang Y, Qiu L, Lu H, Jiang B, Liu C, Peng T, Zhao Y, Wang H, Tsai S, Ngai N, Du L (2015) Mechanisms of soybean roots tolerances to salinity revealed by proteomic and phosphoproteomic comparisons between two cultivars. Mol Cell Proteomics. doi:10.1074/mcp.M115.051961
Polanco MC, Zwiazek JJ, Voicu MC (2008) Responses of ectomycorrhizal American elm (ulmus americana) seedlings to salinity and soil compaction. Plant Soil 308:189–200. doi:10.1007/s11104-008-9619-z
Qin S, Zhang YJ, Yuan B, Xu PY, Xing K, Wang J, Jiang JH (2014) Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte limonium sinense (girard) kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil 374:753–766. doi:10.1007/s11104-014-2072-2
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Routray P, Miller JB, Du L, Oldroyd G, Poovaiah BW (2013) Phosphorylation of S344 in the calmodulin-binding domain negatively affects CCaMK function during bacterial and fungal symbioses. Plant J 76:287–296
Ruiz-Lozano JM, Porcel R, Azcon C, Aroca R (2012) Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J Exp Bot 63:4033–4044. doi:10.1093/Jxb/Ers126
Saur IM, Oakes M, Djordjevic MA, Imin N (2011) Crosstalk between the nodulation signaling pathway and the autoregulation of nodulation in Medicago truncatula. New Phytol 190:865–874. doi:10.1111/j.1469-8137.2011.03738.x
Schmelzer E, Jahnen W, Hahlbrock K (1988) In situ localization of light-induced chalcone synthase mRNA, chalcone synthase, and flavonoid end products in epidermal cells of parsley leaves. PNAS 85:2989–2993
Shaw LJ, Morris P, Hooker JE (2006) Perception and modification of plant flavonoid signals by rhizosphere microorganisms. Environ Microbiol 8:1867–1880. doi:10.1111/j.1462-2920.2006.01141.x
Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, Goodman HM (1995) Analysis of arabidopsis mutants deficient in flavonoid biosynthesis. Plant J 8:659–671
Suzuki T, Honda Y, Mukasa Y, Kim SJ (2006) Characterization of peroxidase in buckwheat seed. Phytochemistry 67:219–224. doi:10.1016/j.phytochem.2005.11.014
Talbi C, Argandona M, Salvador M, Alche JD, Vargas C, Bedmar EJ, Delgado MJ (2013) Burkholderia phymatum improves salt tolerance of symbiotic nitrogen fixation in Phaseolus vulgaris. Plant Soil 367:673–685. doi:10.1007/s11104-012-1499-6
Tejera NA, Soussi M, Lluch C (2006) Physiological and nutritional indicators of tolerance to salinity in chickpea plants growing under symbiotic conditions. Environ Exp Bot 58:17–24. doi:10.1016/j.envexpbot.2005.06.007
Tirichine L, Imaizumi-Anraku H, Yoshida S, Murakami Y, Madsen LH, Miwa H, Nakagawa T, Sandal N, Albrektsen AS, Kawaguchi M, Downie A, Sato S, Tabata S, Kouchi H, Parniske M, Kawasaki S, Stougaard J (2006) Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature 441:1153–1156. doi:10.1038/nature04862
Tran HNN, Brechenmacher L, Aldrich JT, Clauss TR, Gritsenko MA, Hixson KK, Libault M, Tanaka K, Yang F, Yao QM, Pasa-Tolic L, Xu D, Nguyen HT, Stacey G (2012) Quantitative phosphoproteomic analysis of soybean root hairs inoculated with Bradyrhizobium japonicum. Mol Cell Proteomics 11:1140–1155. doi:10.1074/mcp.M112.018028
Turner NC, Colmer TD, Quealy J, Pushpavalli R, Krishnamurthy L, Kaur J, Singh G, Siddique KHM, Vadez V (2013) Salinity tolerance and ion accumulation in chickpea (cicer arietinum L.) subjected to salt stress. Plant Soil 365:347–361. doi:10.1007/s11104-012-1387-0
van Tunen AJ, Koes RE, Spelt CE, van der Krol AR, Stuitje AR, Mol JN (1988) Cloning of the two chalcone flavanone isomerase genes from Petunia hybrida: coordinate, light-regulated and differential expression of flavonoid genes. EMBO J 7:1257–1263
Velarde-Buendia AM, Shabala S, Cvikrova M, Dobrovinskaya O, Pottosin I (2012) Salt-sensitive and salt-tolerant barley varieties differ in the extent of potentiation of the ROS-induced K+ efflux by polyamines. Plant Physiol Biochem 61:18–23. doi:10.1016/j.plaphy.2012.09.002
Wang GP, Zeng HQ, Hu XY, Zhu YY, Chen Y, Shen CJ, Wang HZ, Poovaiah BW, Du LQ (2015) Identification and expression analyses of calmodulin-binding transcription activator genes in soybean. Plant Soil 386:205–221. doi:10.1007/s11104-014-2267-6
Xu ZC, Zhao SJ, Gao HY, Sun S (2014) The salt resistance of wild soybean (glycine soja sieb. et zucc. ZYD 03262) under NaCl stress is mainly determined by Na+ distribution in the plant. Acta Physiol Plant 36:61–70. doi:10.1007/s11738-013-1386-7
Yang LT, Qi YP, Lu YB, Guo P, Sang W, Feng H, Zhang HX, Chen LS (2013) ITRAQ protein profile analysis of citrus sinensis roots in response to long-term boron-deficiency. J Proteome 93:179–206. doi:10.1016/j.jprot.2013.04.025
Yano K, Yoshida S, Muller J, Singh S, Banba M, Vickers K, Markmann K, White C, Schuller B, Sato S, Asamizu E, Tabata S, Murooka Y, Perry J, Wang TL, Kawaguchi M, Imaizumi-Anraku H, Hayashi M, Parniske M (2008) CYCLOPS, a mediator of symbiotic intracellular accommodation. PNAS 105:20540–20545. doi:10.1073/pnas.0806858105
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13:134. doi:10.1186/1471-2105-13-134
Zhang J, Subramanian S, Stacey G, Yu O (2009) Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J 57:171–183. doi:10.1111/j.1365-313X.2008.03676.x
Zhao GM, Han Y, Sun X, Li SH, Shi QM, Wang CH (2015) Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind Crop Prod 64:175–181. doi:10.1016/j.indcrop.2014.10.058
Zhu J, Bie ZL, Li YN (2008) Physiological and growth responses of two different salt-sensitive cucumber cultivars to NaCl stress. Soil Sci Plant Nutr 54:400–407. doi:10.1111/j.1747-0765.2007.00245.x
Acknowledgments
This work was partially supported by grants 31301053 and U1130304 from the National Science Foundation of China, and grant PF14002004014 from Hangzhou Normal University. We are grateful to Mr. Tao Sun. (Hangzhou Normal University) for his kind assistance during the RT-PCR analysis. We extend special thanks to Prof. Liqun Du (Hangzhou Normal University) for manuscript revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Frans J.M Maathuis.
Liqun Qu and Yingying Huang contributed equally to this work.
Electronic supplementary material
Supplemental Table S1
(XLS 46 kb)
Rights and permissions
About this article
Cite this article
Qu, L., Huang, Y., Zhu, C. et al. Rhizobia-inoculation enhances the soybean’s tolerance to salt stress. Plant Soil 400, 209–222 (2016). https://doi.org/10.1007/s11104-015-2728-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2728-6