Abstract
Background and aims
Condensed tannins, a dominant class of plant secondary metabolites, play potentially important roles in plant-soil feedbacks by influencing the soil microbial community. Effects of condensed tannins on the soil microbial community and activity were examined by a short-term tannin-addition experiment under field and laboratory conditions.
Methods
Condensed tannins were extracted from the leaves of a dominant conifer (Dacrydium gracilis) in a tropical montane forest on Mt. Kinabalu, Borneo. The extracted tannins were added to soils beneath the conifer and a dominant broadleaf (Lithocarpus clementianus) to evaluate the dependence of the response to tannin addition on the initial composition of the soil microbial community.
Results
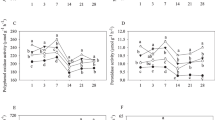
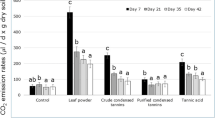
Enzyme activities in the field tannin-addition treatment were lower than in the deionized-water treatment. Carbon and nitrogen mineralization were also inhibited by tannin-addition. The fungi-to-bacteria ratio after tannin-addition was higher compared with the distilled-water treatment in the laboratory experiment.
Conclusions
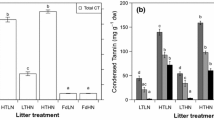
Based on our results, we suggest that the higher concentration of condensed tannins in the leaf tissues of Dacrydium than in those of Lithocarpus is a factor influencing the microbial community and activity. This may have influences on subsequent plant performance, which induces plant-soil feedback processes that can control dynamics of the tropical montane forest ecosystem.






Similar content being viewed by others
References
Aiba S, Kitayama K (1999) Structure, composition and species diversity in an altitude-substrate matrix of rain forest tree communities on Mount Kinabalu, Borneo. Plant Ecol 140:139–157
Aiba S, Kitayama K, Rebin R (2002) Species composition and species-area relationships of trees in nine permanent plots in altitudinal sequences on different geological substrates of Mount Kinabalu. Sabah Parks Nat J 5:7–69
Balser TC, Firestone MK (2005) Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 73:395–415
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bowman WD, Steltzer H, Rosenstiel TN, Cleveland CC, Meier CL (2004) Litter effects of two co-occurring alpine species on plant growth, microbial activity and immobilization of nitrogen. Oikos 104:336–344
Fierer N, Schimel JP, Cates RG, Zou J (2001) Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biol Biochem 33:1827–1839
Frelich LE, Calcote RR, Davis MB, Pastor J (1993) Patch formation and maintenance in an old-growth hemlock-hardwood forest. Ecology 74:513–527
Frostegård A, Tunlid A, Bååth E (1991) Microbial biomass measured as total lipid phosphate in soils of different organic content. J Microbiol Methods 14:151–163
Frostegård A, Bååth E, Tunlid A (1993) Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol Biochem 25:723–730
Frostegârd A, Tunlid A, Bââth E (2011) Use and misuse of PLFA measurements in soils. Soil Biol Biochem 43:1621–1625
Hall SJ, Asner GP, Kitayama K (2004) Substrate, climate, and land use controls over soil N dynamics and N-oxide emissions in Borneo. Biogeochemistry 70:27–58
Hartley SE, Jones CG (1997) Plant chemistry and herbivory: or why the world is green. Plant ecology, 2nd edition edn. Blackwell Science, Cambridge, Mass
Hobbie SE (1992) Effects of plant species on nutrient cycling. TREE 7:336–339
Kanerva S, Smolander A (2008) How do coniferous needle tannins influence C and N transformations in birch humus layer? Eur J Soil Biol 44:1–9
Kanerva S, Kitunen V, Kiikkilä O, Loponen J, Smolander A (2006) Response of soil C and N transformations to tannin fractions originating from Scots pine and Norway spruce needles. Soil Biol Biochem 38:1364–1374
Kardol P, Cornips NJ, van Kempen MML, Bakx-Schotman JMT, van der Putten WH (2007) Microbe-mediated plant-soil feedback causes historical contingency effects in plant community assembly. Ecol Monogr 77:147–162
Kitayama K, Aiba SI, Majalap-Lee N, Ohsawa M (1998) Soil nitrogen mineralization rates of rainforests in a matrix of elevations and geological substrates on Mount Kinabalu, Borneo. Ecol Res 13:301–312
Kitayama K, Aiba S, Takyu M, Majalap N, Wagai R (2004a) Soil phosphorus fractionation and phosphorus-use efficiency of a Bornean tropical montane rain forest during soil aging with podozolization. Ecosystems 7:259–274
Kitayama K, Suzuki S, Hori M, Takyu M, Aiba SI, Majalap-Lee N, Kikuzawa K (2004b) On the relationships between leaf-litter lignin and net primary productivity in tropical rain forests. Oecologia 140:335–339
Kitayama K, Aiba S, Ushio M, Seino T, Fujiki Y (2011) The ecology of porocarps in tropical montane forests of Borneo: distribution, population dynamics, and soil nutrient acquisition. In: Turner BL, Cernusak LA (eds) Ecology of the Podocarpaceae in Tropical Forests, vol 95. Smithsonian contributions to botany. Smithsonian Institution Scholarly Press, Washington DC, pp 101–117
Kraal P, Nierop KGJ, Kaal J, Tietema A (2009) Carbon respiration and nitrogen dynamics in Corsican pine litter amended with aluminium and tannins. Soil Biol Biochem 41:2318–2327
Kraus TEC, Dahlgren RA, Zasoski RJ (2003) Tannins in nutrient dynamics of forest ecosystems—A review. Plant Soil 256:41–66
Kuiters AT (1990) Role of phenolic substances from decomposing forest litter in plant-soil interactions. Acta Bot Neerl 39:329–348
Majuakim L (2005) Influence of foliar polyphenols on dissolved organic nitrogen in the soil water of the tropical montane forests on Mount Kinabalu, Borneo. Master Thesis, Kyoto University, Japan
Meier CL, Bowman WD (2008) Phenolic-rich leaf carbon fractions differentially influence microbial respiration and plant growth. Oecologia 158:95–107
Mentzer JL, Goodman RM, Balser TC (2006) Microbial response over time to hydrologic and fertilization treatments in a simulated wet prairie. Plant Soil 284:85–100
Miki T, Ushio M, Fukui S, Kondoh M (2010) Functional diversity of microbial decomposers facilitates plant coexistence in a model of plant-microbe-soil feedback. Proc Natl Acad Sci U S A 107:14251–14256
Neill C, Piccolo MC, Cerri CC, Steudler PA, Melillo JM, Brito M (1997) Net nitrogen mineralization and net nitrification rates in soils following deforestation for pasture across the southwestern Brazilian Amazon Basin landscape. Oecologia 110:243–252
Nierop KGJ, Verstraten JM, Tietema A, Westerveld JW, Wartenbergh PE (2006) Short- and long-term tannin induced carbon, nitrogen and phosphorus dynamics in Corsican pine litter. Biogeochemistry 79:275–296
Northup RR, Yu Z, Dahlren RA, Vogt KA (1995) Polyphenol control of nitrogen release from pine litter. Nature 377:227–229
Piccolo MC, Neill C, Cerri CC (1994) Net nitrogen mineralization and net nitrification along a tropical forest-to-pasture chronosequence. Plant Soil 162:61–70
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team (2009) nlme: Linear and Nonlinear Mixed Effects of Models. R package version 3.1
Porter LJ, Hrstich LN, Chan BG (1986) The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25:223–230
R Development Core Team (2011) R: A language and environment for statistical computing. Vienna, Austria
Ratledge C, Wilkinson SG (1988) Microbial lipids. Academic, London
Scalbert A (1991) Antimicrobial properties of tannins. Phytochemistry 30:3875–3883
Schimel JP, Cates RG, Ruess R (1998) The role of balsam poplar secondary chemicals in controlling soil nutrient dynamics through succession in the Alaskan taiga. Biogeochemistry 42:221–234
Smolander A, Kanerva S, Adamczyk B, Kitunen V (2012) Nitrogen transformations in boreal forest soils-does composition of plant secondary compounds give any explanations? Plant Soil 350:1–26
Strickland MS, Lauber C, Fierer N, Bradford MA (2009) Testing the functional significance of microbial community composition. Ecology 90:441–451. doi:doi:10.1890/08-0296.1
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Ushio M, Adams JM (2011) A meta-analysis of the global distribution pattern of condensed tannins in woody tree leaves. Open Ecol J 4:18–23
Ushio M, Wagai R, Balser TC, Kitayama K (2008) Variations in the soil microbial community composition of a tropical montane forest ecosystem: does tree species matter? Soil Biol Biochem 40:2699–2702
Ushio M, Miki T, Kitayama K (2009) Phenolic control of plant nitrogen acquisition through the inhibition of soil microbial decomposition processes: a plant-microbe competition model. Microbes Environ 24:180–187
Ushio M, Kitayama K, Balser TC (2010a) Tree species effects on soil enzyme activities through effects on soil physicochemical and microbial properites in a tropical montane forest on Mt. Kinabalu, Borneo. Pedobiologia 53:227–233
Ushio M, Kitayama K, Balser TC (2010b) Tree species-mediated spatial patchiness of the composition of microbial community and physicochemical properties in the topsoils of a tropical montane forest. Soil Biol Biochem 42:1588–1595
van der Putten WH, Bardgett RD, de Ruiter PC, Hol WHG, Meyer KM, Bezemer TM, Bradford MA, Christensen S, Eppinga MB, Fukami T, Hemerik L, Molofsky J, Schädler M, Scherber C, Strauss SY, Vos M, Wardle DA (2009) Empirical and theoretical challenges in aboveground-belowground ecology. Oecologia 161:1–14
Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
White DC, Ringelberg DB (1998) Signature lipid biomarker analysis. Techniques in microbial ecology. Oxford University Press, New York
White DC, Davis WM, Nickels JS, King JD, Bobbie RJ (1979) Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 40:51–62
Wood SN (2004) Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc 99:673–686
Wurzburger N, Hendrick RL (2009) Plant litter chemistry and mycorrhizal roots promote a nitrogen feedback in a temperate forest. J Ecol 97:528–536
Yao H, He Z, Wilson MJ, Campbell CD (2000) Microbial biomass and community structure in a sequence of soils with increasing fertility and changing land use. Microb Ecol 40:223–237
Zelles L, Rackwitz R, Bai QY, Beck T, Beese F (1995) Discrimination of microbial diversity by fatty acid profiles of phospholipids and lipopolysaccharides in differently cultivated soils. Plant Soil 170:115–122
Acknowledgements
We thank Prof. Tohru Mitsunaga and Dr. Hiroyuki Takemoto for their support with the tannin extraction procedure. We also thank staffs of the Sabah Parks for their support and permission throughout the course of our research and Dr. Sizuo Suzuki for providing us with the data on leaf phenolics. We also thank Dr. Harry Read, Mr. Kevin Budsberg, Dr. Chao Liang and members of the Balser Lab at the University of Wisconsin-Madison for their support with lipid analysis. This research was supported by a grant-in-aid (MESSC 19380010) to K.K. and in part by Global COE program A06 to Kyoto University. M.U. is supported by JSPS Research Fellowship for Young Scientists (21–1526 and 23–586).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 80 kb)
Rights and permissions
About this article
Cite this article
Ushio, M., Balser, T.C. & Kitayama, K. Effects of condensed tannins in conifer leaves on the composition and activity of the soil microbial community in a tropical montane forest. Plant Soil 365, 157–170 (2013). https://doi.org/10.1007/s11104-012-1365-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1365-6