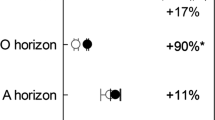
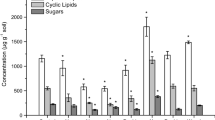
Abstract
Two major groups of plant secondary compounds, phenolic compounds and terpenes, may according to current evidence mediate changes in soil C and N cycling, but their exact role and importance in boreal forest soils are largely unknown. In this review we discuss the occurrence of these compounds in forest plants and soils, the great challenges faced when their concentrations are measured, their possible effects in regulating soil C and N transformations and finally, we attempt to evaluate their role in connection with certain forest management practices. In laboratory experiments, volatile monoterpenes, in the concentrations found in the coniferous soil atmosphere, have been shown to inhibit net nitrogen mineralization and nitrification; they probably provide a C source to part of the soil microbial population but are toxic to another part. However, there is a large gap in our knowledge of the effects of higher terpenes on soil processes. According to results from laboratory experiments, an important group of phenolic compounds, condensed tannins, may also affect microbial processes related to soil C and N cycling; one mechanism is binding of proteins and certain other organic N-containing compounds. Field studies revealed interesting correlations between the occurrence of terpenes or phenolic compounds and C or net N mineralization in forest soils; in some cases these correlations point in the same direction as do the results from laboratory experiments, but not always. Different forest management practices may result in changes in both the quantity and quality of terpenes and phenolic compounds entering the soil. Possible effects of tree species composition, clear-cutting and removal of logging residue for bioenergy on plant secondary compound composition in soil are discussed in relation to changes observed in soil N transformations.









Similar content being viewed by others
References
Adamczyk B, Godlewski M, Zimny J, Zimny A (2008a) Wheat (Triticum aestivum) seedlings secrete proteases from roots and, after protein addition, grow well on medium without inorganic nitrogen. Plant Biol 10:718–724
Adamczyk B, Kitunen V, Smolander A (2008b) Protein precipitation by tannins in soil organic horizon and vegetation in relation to tree species. Biol Fertil Soils 45:55–64
Adamczyk B, Godlewski M, Smolander A, Kitunen V (2009a) Degradation of proteins by enzymes exuded by Allium porrum roots—a potential important plant strategy for acquiring organic nitrogen. Plant Physiol Biochem 47:919–925
Adamczyk B, Kitunen V, Smolander A (2009b) Polyphenol oxidase, tannase and proteolytic activity in relation to tannin concentration in the soil organic horizon under silver birch and Norway spruce. Soil Biol Biochem 41:2085–2093
Adamczyk B, Smolander A, Kitunen V, Godlewski M (2010) Proteins as nitrogen source for plants. A short story about exudation of proteases by plant roots. Plant Signal Behav 5:1–3
Adamczyk B, Adamczyk S, Smolander A, Kitunen V (2011a) Tannic acid and condensed tannins from Norway spruce can precipitate various organic nitrogen compounds. Soil Biol Biochem 43:628–637
Adamczyk S, Adamczyk B, Kitunen V, Smolander A (2011b) Influence of diterpenes (colophony and abietic acid) and a triterpene (beta-sitosterol) on net N mineralization, net nitrification, soil respiration, and microbial biomass in birch soil. Biol Fertil Soils, in press. doi:10.1007/s00374-010-0529-x
Aderiye BI, Ogundana SK, Adesanya SA, Roberts MF (1989) The effects of β-sitosterol on spore germination and germ-tube elongation of Aspergillus niger and Botryodiplodia theobromae. Int J Food Microbiol 8:73–78
Amaral JA, Knowles R (1998) Inhibition of methane consumption in forest soils by monoterpenes. J Chem Ecol 24:723–734
Amaral JA, Ekins A, Richards SR, Knowles R (1998) Effect of selected monoterpenes on methane oxidation, denitrification, and aerobic metabolism by bacteria in pure culture. Appl Environ Microbiol 64:520–525
An J-H, Dultz S (2007) Adsorption of tannic acid on chitosan-montmorillonite as a function of pH surface charge properties. Appl Clay Sci 36:256–264
Appel HM, Govenor HL, D’Ascenzo M, Siska E, Schultz JC (2001) Limitations of Folin assays of foliar phenolics in ecological studies. J Chem Ecol 27:761–778
Asensio D, Owen SM, Llusia J, Penuelas J (2008) The distribution of volatile isoprenoids in the soil organic horizons around Pinus halepensis trees. Soil Biol Biochem 40:2937–2947
Back EL, Ekman R (2000) Definitions of wood resin and its components. In: Back EL, Allen LH (eds) Pitch control, wood resin and deresination. TAPPI, Atlanta, pp vii–xi
Bäck J, Aaltonen H, Hellén H, Kajos MK, Patokoski J, Taipale R, Pumpanen J, Heinonsalo J (2010) Variable emissions of microbial volatile organic compounds (MVOCs) from root associated fungi isolated from Scots pine. Atmos Environ 44:3651–3659
Baldwin IT, Olson RK, Reiners WA (1983) Protein binding phenolics and the inhibition of nitrification in subalpine balsam fir soils. Soil Biol Biochem 15:419–423
Bending GD, Read DJ (1996) Nitrogen mobilization from protein polyphenol complex by ericoid and ectomycorrhizal fungi. Soil Biol Biochem 28:1603–1612
Blum U, Shafer SR (1988) Microbial populations and phenolic acids in soil. Soil Biol Biochem 20:793–800
Box JD (1981) Investigation of the Folin-Ciocalteu phenol reagent for the determination of polyphenolic substances in natural waters. Water Res 17:511–525
Bradley RL, Titus BD, Preston CP (2000) Changes to mineral N cycling and microbial communities in black spruce humus after additions of (NH4)2SO4 and condensed tannins extracted from Kalmia angustifolia and balsam fir. Soil Biol Biochem 32:1227–1240
Bremner JM, McCarty GW (1988) Effects of terpenoids on nitrification in soil. Soil Sci Soc Am J 52:1630–1633
Bremner JM, McCarty GW (1996) Inhibition of nitrification in soil by allelochemicals derived from plants and plant residues. Soil Biochem 8:181–218
Chang M-Y, Juang R-S (2004) Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and active clay. J Colloid Interface Sci 278:18–25
Close DC, McArthur C (2002) Rethinking the role of many plant phenolics—protection from photodamage not herbivores? Oikos 99:166–172
Dahlgren RA, Driscoll CT (1994) The effects of whole-tree clear-cutting on soil processes at the Hubbard Brook Experimental Forest, New Hampshire, USA. Plant Soil 158:239–262
De Boer W, Kester RA (1996) Variability of nitrification potentials in patches of undergrowth vegetation in primary Scots pine stands. For Ecol Manage 86:97–103
Dev S (1989) Terpenoids. In: Rowe JW (ed) Natural products of woody plants II: chemicals extraneous to lignocellusic cell wall. Springer, Berlin, pp 691–807
Dijkstra EF, Boon JJ, Van Mourik JM (1998) Analytical pyrolysis of a soil profile under Scots pine. Eur J Soil Sci 49:295–304
Ekman R, Holmbom B (2000) The chemistry of wood resin. In: Back EL, Allen LH (eds) Pitch control, wood resin and deresination. TAPPI, Atlanta, pp 37–76
Fierer N, Schimel JP, Gates RG, Zou J (2001) The influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biol Biochem 33:1827–1839
Gallet C, Lebreton P (1995) Evolution of phenolic patterns in plants and associated litters and humus of a mountain forest ecosystem. Soil Biol Biochem 27:157–165
Gärdenäs AI, Ågren GI, Bird JA, Clarholm M, Hallin S, Ineson P, Kättener T, Knicker H, Nilsson I, Näsholm T, Ogle S, Paustian K, Persson T, Stendahl J (2011). Knowledge gaps in soil carbon and nitrogen interactions—from molecular to global scale. Soil Biol Biochem 43:702–717
Gershenzon J, Dudareva N (2007) The function of terpene natural products in the natural world. Nat Chem Biol 3:408–414
Gil F, De la Iglesia R, Mendoza L, Gonzales B, Wilkens M (2006) Soil bacteria are differentially affected by the resin of the medicinal plant Pseudognaphalium vira vira and its main component kaurenoic acid. Microb Ecol 52:10–18
Grayston S, Prescott C (2005) Microbial communities in forest floors under four tree species in coastal British Columbia. Soil Biol Biochem 37:1157–1167
Gundale MJ, Sverker J, Albrectsen BR, Nilsson M-C, Wardle DA (2010) Variation in protein complexation capacity among and within six plant species across a boreal forest chronosequence. Plant Ecol 211:253–266
Hagerman AE (1987) Radial diffusion method for determining tannin in plant extracts. J Chem Ecol 13:437–449
Hagerman AE, Butler LG (1978) Protein precipitation method for quantitative determination of tannins. J Agr Food Chem 26:809–812
Hagerman AE, Butler LG (1981) The specificity of proanthocyanidin-protein interactions. J Biol Chem 256:4494–4497
Hagerman AE, Robbins CT (1987) Implications of soluble tannin-protein complexes for tannin analysis and plant defense mechanisms. J Chem Ecol 13:1243–1259
Hagerman AE, Rice ME, Ritchard NT (1998) Mechanisms of protein precipitation for two tannins, pentagallyol glucose and epicatechin16 (4 → 8) catechin (procyanidin). J Agric Food Chem 46:2590–2595
Halvorson JJ, Gonzales JM (2008) Tannic acid reduces recovery of water-soluble carbon and nitrogen from soil and affects the composition of Bradford-reactive soil protein. Soil Biol Biochem 40:186–197
Halvorson JJ, Gonzales JM, Hagerman AE, Smith JL (2009) Sorption of tannin and related phenolic compounds and effects on soluble-N in soil. Soil Biol Biochem 41:2002–2010
Handayanto E, Giller KE, Cadisch G (1997) Regulating N release from legume tree prunings by mixing residues of different quality. Soil Biol Biochem 29:1417–1426
Harborne JB (1997) Role of phenolic secondary metabolites in plants and their degradation in nature. In: Cadisch G, Giller KE (eds) Driven by nature: plant litter quality and decomposition. CAB International, pp 67–74
Hartzfeld PW, Forkner R, Hunter MD, Hagerman AE (2002) Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J Agr Food Chem 50:1785–1790
Haslam E (1989) Plant polyphenols—vegetable tannins revisited. In: Phillipson JD, Ayres DC, Baxter H (eds) Chemistry and pharmacology of natural products. Cambridge University Press, Cambridge, p 230
Hättenschwiler S, Vitousek PM (2000) The role of polyphenols in terrrestrial ecosystem nutrient cycling. TREE 15:238–243
Hernes PJ, Hedges JI (2004) Tannin signatures of barks, needles, leaves, cones, and wood at the molecular level. Geochim Cosmochim Acta 68:1293–1307
Howard PJA, Howard DM (1993) Ammonification of complexes prepared from gelatin and aqueous extracts of leaves and freshly-fallen litter of trees on different soil types. Soil Biol Biochem 25:1249–1256
Insam H, Seewald MSA (2010) Volatile organic compounds (VOCs) in soils. Biol Fertil Soils 46:199–213
Jacobson S, Kukkola M, Mälkönen E, Tveite B, Möller G (2000) Growth response of coniferous stands to whole-tree harvesting in early thinnings. Scand J For Res 11:50–59
Joanisse GD, Bradley RL, Preston CM, Munson AD (2007) Soil enzyme inhibition by condensed tannins may drive ecosystem structure and processes: the case of Kalmia angustifolia. New Phytol 175:535–546
Joanisse GD, Bradley RL, Preston CM (2008) Do late-successional tannin-rich plant communities occurring on highly acidic soils increase the DON/DIN ratio? Biol Fertil Soils 44:903–907
Juntheikki M-R (1999) Chemoecological role of tannins in woody plants. Dissertation. University of Joensuu, Finland
Juntheikki M-R, Julkunen-Tiitto R (2000) Inhibition of β-glucosidase and esterase by tannins from Betula, Salix, and Pinus species. J Chem Ecol 26:1151–1165
Kainulainen P, Holopainen JK (2002) Concentrations of secondary metabolites in Scots pine needles at different stages of decomposition. Soil Biol Biochem 34:37–42
Kalina M, Pease DC (1977) The preservation of ultrastructure in saturated phosphatidyl colines by tannic acid in model systems and type II pneumocytes. J Cell Biol 74:726–741
Kanerva S, Smolander A (2007) Microbial activities in forest floor layers under silver birch, Norway spruce and Scots pine. Soil Biol Biochem 39:1459–1467
Kanerva S, Smolander A (2008) How do coniferous needle tannins influence C and N transformations in birch soil. Eur J Soil Biol 44:1–9
Kanerva S, Kitunen V, Kiikkilä O, Loponen J, Smolander A (2006) Response of soil C and N transformations to tannin fractions extracted from Norway spruce and Scots pine needles. Soil Biol Biochem 38:1364–1374
Kanerva S, Kitunen V, Loponen J, Smolander A (2008) Phenolic compounds and terpenes in soil organic horizon layers under silver birch, Norway spruce and Scots pine. Biol Fertil Soils 44:547–556
Ketola RA, Kiuru JT, Kotiaho T, Kitunen V, Smolander A (2010) Feasibility of membrane inlet mass spectrometry for on-site screening of volatile monoterpenes and monoterpene alcohols in forest soil atmosphere. Boreal Environ Res 16:36–46
Kiikkilä O, Kitunen V, Smolander A (2005) Degradability of dissolved organic carbon and nitrogen in relation to tree species. FEMS Microbiol Ecol 53:33–40
Kiikkilä O, Kitunen V, Smolander A (2006) Dissolved soil organic matter from surface organic horizons under birch and conifers: degradation in relation to chemical characteristics. Soil Biol Biochem 38:737–746
Knicker H (2011) Soil organic N—an under-rated player for C sequestration in soils? Soil Biol Biochem 43:1118–1129
Kraal P, Nierop KGJ, Kaal J, Tietema A (2009) Carbon respiration and nitrogen dynamics in Corsican pine litter amended with aluminium and tannins. Soil Biol Biochem 41:2318–2327
Kranabetter JM, Banner A (2000) Selected biological and chemical properties of forest floors across bedrock types on the north coast of British Columbia. Can J For Res 30:971–981
Kraus TEC, Dahlgren RA, Zasoski RJ (2003) Tannins in nutrient dynamics of forest ecosystems—a review. Plant Soil 256:41–66
Kraus TEC, Zasoski RJ, Dahlgren RA, Horwath WR, Preston CM (2004a) Carbon and nitrogen dynamics in a forest soil amended with purified tannins from different plant species. Soil Biol Biochem 36:309–321
Kraus TEC, Zasoski RJ, Dahlgren RA (2004b) Fertility and pH effects on polyphenol and condensed tannin concentrations in foliage and roots. Plant Soil 262:95–109
Kumar R, Horigome T (1986) Fractionation, characterization, and protein-precipitating capacity of the condensed tannins from Robinia pseudo acacia L. leaves. J Agr Food Chem 34:487–489
Langenheim JH (1994) Higher plant terpenoids: a phytocentric overview of their ecological roles. J Chem Ecol 20:1223–1280
Laothawornkitkul J, Taylor JE, Paul ND, Hewitt CN (2009) Biogenic volatile organic compounds in the Earth System. Tansley Review New Phytol 183:27–51
Leff J, Fierer N (2008) Volatile organic compound (VOC) emissions from soil and litter samples. Soil Biol Biochem 40:1629–1636
Lenoir L, Bengtson J, Persson T (1999) Effects of coniferous resin on fungal biomass and mineralization processes in wood ant nest materials. Biol Fertil Soils 30:251–257
Lin C, Owen SM, Peneelas J (2007) Volatile organic compounds in the roots and rhizosphere of Pinus spp. Soil Biol Biochem 39:951–960
Lipson D, Näsholm T (2001) The unexpected versatility of plants: organic nitrogen use and availability in terrestrial ecosystems. Oecologia 128:305–316
Lodhi MAK, Killingbeck KT (1980) Allelopathic inhibition of nitrification and nitrifying bacteria in a Ponderosa pine (Pinus ponderosa Dougl) community. Amer J Bot 67:1423–1429
Lorenz K, Preston CM, Raspe S, Morrison IK, Ferger KH (2000) Litter decomposition and humus characteristics in Canadian and German spruce ecosystems: information from tannin analysis and 13C CPMAS NMR. Soil Biol Biochem 32:779–792
Ludley KE, Robinson CH, Jickells S, Chamberlain PM, Whitaker J (2008) Differential response of ectomycorrhizal and saprotrophic fungal mycelium from coniferous forest soil to selected monoterpenes. Soil Biol Biochem 40:669–678
Ludley KE, Jickells SM, Chamberlain PM, Whitaker J, Robinson CH (2009a) Distribution of monoterpenes between organic resources in upper soil horizons under monocultures of Picea abies, Picea sithensis and Pinus sylvestris. Soil Biol Biochem 41:1050–1059
Ludley KE, Robinson CH, Jickells S, Chamberlain PM, Whitaker J (2009b) Potential for monoterpenes to affect ectomycorrhizal and saprophytic fungal activity in coniferous forest is revealed by a novel experimental system. Soil Biol Biochem 41:117–124
Madricht MD, Jordan LM, Lindroth RL (2007) Interactive effects of condensed tannin and cellulose additions on soil respiration. Can J For Res 37:2063–2067
Maie N, Behrens A, Knicker H, Kögel-Knabner I (2003) Changes in the structure and protein binding ability of condensed tannins during decomposition of fresh needles and leaves. Soil Biol Biochem 35:577–589
Manninen A-M, Utriainen J, Holopainen T, Kainulainen P (2002) Terpenoids in the wood of Scots pine and Norway spruce seedlings exposed to ozone at different nitrogen availability. Can J For Res 32:2140–2145
Martikainen PJ (1996) Microbial processes in boreal forest soils as affected by forest management practices and atmospheric stress. Soil Biochem 9:195–232
Maurer DM, Kolb S, Haumaier L, Borken L (2008) Inhibition of atmospheric methane oxidation by monoterpenes in Norway spruce and European beech soils. Soil Biol Biochem 40:3014–3020
McAllister TA, Martinez T, Bae DH, Muir AD, Yanke LJ, Jones GA (2005) Characterization of condensed tannins purified from legume forages: chromophore production, protein precipitation, and inhibitory effects on cellulose digestion. J Chem Ecol 31:2049–2068
McCarty GW, Bremner JM (1986) Effects of phenolic compounds on nitrification in soil. Soil Sci Soc Am 50:920–923
Melin E, Krupa S (1971) Studies on ectomycorrhizae of pine II. Growth inhibition of mycorrhizal fungi by volatile organic constituents of Pinus silvestris (Scots pine) roots. Physiol Plant 25:337–340
Menyailo OV, Hungate B, Zech W (2002) The effect of single tree species on soil microbial activities related to C and N cycling in the Siberian artificial afforestation experiment. Tree species and soil microbial activities. Plant Soil 242:183–196
Merilä P, Smolander A, Strömmer R (2002) Soil nitrogen transformations along a primary succession transect on the land-uplift coast in western Finland. Soil Biol Biochem 34:373–385
Misra G, Pavlostathis SG, Perdue EM, Araujo R (1996) Aerobic biodegradation of selected monoterpenes. Appl Microbiol Biotechnol 45:831–838
Mole S, Waterman PG (1987) A critical analysis of techniques for measuring tannins in ecological studies. II. Techniques for biochemically defining tannins. Oecologia 72:148–156
Mutabaruka R, Hairiah K, Cadisch G (2007) Microbial degradation of hydrolyzable and condensed tannin polyphenol-protein complexes in soils from different land-use histories. Soil Biol Biochem 39:1479–1492
Nannipieri P, Eldor P (2009) The chemical and biological characterization of soil N and its biotic components. Soil Biol Biochem 41:2357–2369
Napierała-Filipiak A, Werner A, Mardarowicz M, Gawdizik J (2002) Concentrations of terpenes in mycorrhizal roots of Scots pine (Pinus sylvestris L.) seedlings grown in vitro. Acta Physiol Plant 24:137–143
Näsholm T, Ekblad A, Nordin A, Giesler R, Högberg M, Högberg P (1998) Boreal forest plants take up organic nitrogen. Nature 392:914–916
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. Tansley Review New Phytol 182:31–48
Nierop KGJ, Verstraten JM (2006) Fate of tannins in Corsican pine litter. J Chem Ecol 32:2709–2719
Nierop KGJ, Preston CM, Kaal J (2005) Thermally assisted hydrolysis and methylation of purified tannins from plants. Anal Chem 77:5604–5614
Nierop KGJ, Preston CM, Verstraten JM (2006a) Linking the B ring hydroxylation pattern of condensed tannins to C, N and P mineralization. A case study using four tannins. Soil Biol Biochem 38:2794–2802
Nierop KGJ, Verstraten JM, Tietema A, Westerveld JW, Wartenbergh PE (2006b) Short- and long-term tannin induced carbon, nitrogen and phosphorus dynamics in Corsican pine litter. Biogeochemistry 79:275–296
Norris CE, Preston CM, Hogg KE, Titus BD (2011) The influence of condensed tannin structure on rate of microbial mineralization and reactivity to chemical assays. J Chem Ecol 37:311–319
Northup RR, Dahlgren RA, Zengshou Y (1995a) Intraspecific variation of conifer phenolic concentration on a marine terrace soil acidity gradient; a new interpretation. Plant Soil 171:255–262
Northup RR, Yu Z, Dahlgren RA, Voigt KA (1995b) Polyphenol control of nitrogen release from pine litter. Nature 377:227–229
Northup RR, Dahlgren RA, McColl JG (1998) Polyphenols as regulators of plant-litter-soil interactions in Northern California’s pygmy forest: a positive feedback? Biogeochemistry 42:189–220
Obst JR (1998) Special (secondary) metabolites from wood. In: Bruce A, Palfreyman JW (eds) Forest products biotechnology. Taylor & Francis, pp 151–165
Paavolainen L, Smolander A (1998) Nitrification and denitrification in soil from a clear-cut Norway spruce (Picea abies) stand. Soil Biol Biochem 30:775–778
Paavolainen L, Kitunen V, Smolander A (1998) Inhibition of nitrification by monoterpenes. Plant Soil 205:147–154
Parry MAJ, Andralojc PJ, Mitchell RAC, Madgwick PJ, Keys AJ (2003) Manipulation of Rubisco: the amount, activity, function and regulation. J Exp Bot 54:1321–1333
Persson J, Näsholm T (2001) Amino acid uptake: a widespread ability among boreal forest plants. Ecol Lett 4:434–438
Persson T, Wirén A (1995) Nitrogen mineralization and potential nitrification at different depths in acid forest soil. Plant Soil 168–169:55–65
Persson J, Högberg P, Ekblad A, Högberg M, Nordgren A, Näsholm T (2003) Nitrogen acquisition from inorganic and organic sources by boreal forest plants in the field. Oecologia 137:252–257
Piatek KB, Lee Allen H (1999) Nitrogen mineralization in a pine plantation fifteen years after harvesting and site preparation. Soil Sci Soc Am J 63:990–998
Popova M, Trusheva B, Gyosheva M, Tsvetkova I, Bankova V (2009) Antibacterial triterpenes from the threatened wood-decay fungus Fomitopsis rosea. Fitoterapia 80:263–266
Prescott CE, Vesterdal L (2005) Effects of British Columbia tree species on forest floor chemistry. In: Binkley D, Menyailo O (eds) Tree species effects on soils: implications for global change. Springer, Netherlands, pp 17–29
Preston C, Bhatti J, Flanagan L, Norris C (2006) Stocks, chemistry and sensitivity to climate change of dead organic matter along the Canadian Boreal Forest Transect Case Study. Clim Change 74:223–251
Priha O, Smolander A (1999) Nitrogen transformations in soil under Pinus sylvestris, Picea abies and Betula pendula at originally similar forest sites. Soil Biol Biochem 31:965–977
Priha O, Grayston SJ, Hiukka R, Pennanen T, Smolander A (2001) Microbial community structure and characteristics of the organic matter in soils under Pinus sylvestris, Picea abies and Betula pendula at two forest sites. Biol Fertil Soils 33:17–24
Rice EL, Pancholy SK (1974) Inhibition of nitrification by climax ecosystems. III. Inhibitors other than tannins. Am J Bot 61:1095–1103
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Schimel JP, Van Cleve KV, Cates RG, Thomas TP, Reichardt PB (1996) Effects of balsam poplar (Populus balsamifera) tannins and low molecular weight phenolics on microbial activity in taiga floodplain soil: implications for changes in N cycling during succession. Can J Bot 74:84–90
Schimel JP, Cates RG, Ruess R (1998) The role of balsam poplar secondary chemicals in controlling soil nutrient dynamics through succession in the Alaskan taiga. Biogeochemistry 42:221–234
Schofield P, Mbugua DM, Pell AN (2001) Analysis of condensed tannins: a review. Anim Feed Sci Technol 91:21–40
Schulten H-R, Schnitzer M (1998) The chemistry of soil organic nitrogen: a review. Biol Fertil Soils 26:1–15
Senwo ZN, Tabatabai MA (1998) Amino acid composition of soil organic matter. Biol Fertil Soils 26:235–242
Smania EFA, Monache FD, Smania A, Yunes RA, Cuneo RS (2003) Antifungal activity of sterols and triterpenes isolated from Ganoderma annulare. Fitoterapia 74:375–377
Smolander A, Kitunen V (2011) Comparison of tree species effects on microbial C and N transformations and dissolved organic matter properties in boreal forest floors. Appl Soil Ecol, in press. doi:10.1016/j.apsoil.2011.05.002
Smolander A, Kitunen V (2002) Soil microbial activities and characteristics of dissolved organic C and N in relation to tree species. Soil Biol Biochem 34:651–660
Smolander A, Priha O, Paavolainen L, Steer J, Mälkönen E (1998) Nitrogen and carbon transformations before and after clear-cutting in repeatedly N-fertilized and limed forest soil. Soil Biol Biochem 30:477–490
Smolander A, Kukkola M, Helmisaari H-S, Mäkipää R, Mälkönen E (2000). Functioning of forest ecosystems under nitrogen loading. In: Mälkönen E (ed) Forest condition in a changing environment—the Finnish case. Forestry Sciences, vol 65. Kluwer Academic Publishers, pp 229–247
Smolander A, Kitunen V, Mälkönen E (2001) Dissolved soil organic nitrogen and carbon in a Norway spruce stand and an adjacent clear-cut. Biol Fertil Soils 33:190–196
Smolander A, Loponen J, Suominen K, Kitunen V (2005) Organic matter characteristics and C and N transformations in the humus layer under two tree species, Betula pendula and Picea abies. Soil Biol Biochem 37:1309–1318
Smolander A, Ketola R, Kotiaho T, Kanerva S, Suominen K, Kitunen V (2006) Volatile monoterpenes in soil microair under birch and conifers: effects on soil N transformations. Soil Biol Biochem 38:3436–3442
Smolander A, Levula T, Kitunen V (2008) Response of litter decomposition and soil C and N transformations in a Norway spruce thinning stand to removal of logging residues. For Ecol Manage 256:1080–1086
Smolander A, Kitunen V, Tamminen P, Kukkola M (2010a) Removal of logging residue in Norway spruce thinning stands: long-term changes in organic layer properties. Soil Biol Biochem 42:1122–1228
Smolander A, Kitunen V, Tamminen P, Kukkola M (2010b) Response of soil organic layer characteristics to different amounts of logging residue in a Scots pine thinning stand. Geophysical Research Abstracts 12, EGU2010-3222
Stahl PD, Parkin TB (1996) Microbial production of volatile organic compounds in soil microcosms. Soil Sci Soc Am J 60:821–828
Stark S, Julkunen-Tiitto R, Kumpula J (2007) Ecological role of reindeer summer browsing in the mountain birch (Betula pubescens ssp. czerepanovii) forests: effects of plant defense, litter decomposition, and soil nutrient cycling. Oecology 151:486–498
Strömvall A-M, Peterson G (2000) Volatile terpenes emitted to air. In: Back EL, Allen LH (eds) Pitch control, wood resin and deresination. TAPPI, Atlanta, pp 77–99
Suominen K, Kitunen V, Smolander A (2003) Characteristics of dissolved organic matter and phenolic compounds in forest soils under silver birch (Betula pendula), Norway spruce (Picea abies) and Scots pine (Pinus sylvestris). Eur J Soil Sci 54:287–293
Talbot JM, Finzi AC (2008) Differential effects of sugar maple, red oak, and hemlock tannins on carbon and nitrogen cycling in temperate forest soils. Oecologia 155:583–592
Tamm CO, Holmen B, Popovic B, Wiklander G (1974) Leaching of plant nutrients from soils as a consequence of forest operations. Ambio 3:211–221
Tamminen P (2000) Soil factors. In: Mälkönen E. (ed) Forest condition in a changing environment—the Finnish case. Forestry Sciences, vol. 65. Kluwer Academic Publishers, pp 72–86
Thoss V, Shevtsova A, Nilsson M-C (2004) Environmental manipulation treatment effects on the reactivity of water-soluble phenolics in a subalpine tundra ecosystem. Plant and Soil 259:355–356
Uusitalo M, Kitunen V, Smolander A (2008) Response of C and N transformations in birch soil to coniferous resin volatiles. Soil Biol Biochem 40:2643–2649
Valachovic YS, Caldwell BA, Cromack K, Griffiths RP (2004) Leaf litter chemistry controls on decomposition of Pacific Northwest trees and woody shrubs. Can J For Res 34:2131–2147
Van Roon A, Parsons JR, Krap L, Govers HAJ (2005) Fate and transport of monoterpenes through soils. Part II. Calculation of the effect of soil temperature, water saturation and organic carbon content. Chemosphere 61:129–138
Verkaik E, Jongkind AE, Berendse F (2006) Short-term and long-term effects of tannins on nitrogen mineralization and litter decomposition in kauri (Agathis australis (D. Don) Lindl) forests. Plant Soil 287:337–345
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: How can it occur? Biogeochem 13:87–115
Vitousek PM, Matson PA (1984) Mechanisms of nitrogen retention in forest ecosystems: a field experiment. Science 225:51–52
Ward BB, Courtney KJ, Langenheim JH (1997) Inhibition of Nitromonas europaea by monoterpenes from coastal redwood (Sequoia sempervirens) in whole-cell studies. J Chem Ecol 23:2583–2598
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites. In: Lawton JH, Likens GE (eds) Methods in ecology. Blackwell Scientific Publications, Oxford, pp 92–95
Weidenhamer JD, Macias FA, Fischer NH, Williamson GB (1993) Just how insoluble are monoterpenes? J Chem Ecol 19:1779–1807
Werner A, Napierała-Filipiak A, Mardarowich M, Gawdzik J (2004) The effects of heavy metals, contents of nutrients and inoculation with mycorrhizal fungi on the level of terpenoids in roots of Pinus sylvestris seedlings. Acta Physiol Plantarum 26:187–196
White CS (1986) Volatile and water-soluble inhibitors of nitrogen mineralisation and nitrification in a ponderosa pine ecosystem. Biol Fertil Soils 2:97–104
White CS (1988) Nitrification inhibition by monoterpenoids: theoretical mode of action based on molecular structures. Ecology 69:1631–1633
White CS (1991) The role of monoterpenes in soil nitrogen cycling processes in ponderosa pine. Results from laboratory bioassays and field studies. Biogeochem 12:43–68
White CS (1994) Monoterpenes: their effects on ecosystem nutrient cycling. J Chem Ecol 20:1381–1406
Wilt FM, Miller GC, Everett RL (1988) Monoterpene concentrations in litter and soil of single-leaf pinyon woodlands of the Western Great Basin. Great Basin Nat 48:228–231
Wilt FM, Miller GC, Everett RL, Hackett M (1993a) Monoterpene concentrations in fresh, senescent, and decaying foliage of single-leaf pinyon (Pinus monofylla Torr. & Frem.: Pinaceae) from the Western Great Basin. J Chem Ecol 19:185–194
Wilt FM, Miller GC, Everett RL (1993b) Measurement of monoterpene hydrocarbon levels in vapor phase surrounding single-leaf pinyon (Pinus monophylla Torr. & Frem.: Pinaceae) understory litter. J Chem Ecol 19:1417–1428
Wu T, Sharda JN, Koide RT (2003) Exploring interactions between saprotrophic microbes and ectomycorrhizal fungi using protein-tannin complex as N source by red pine (Pinus resinosa). New Phytol 159:131–139
Acknowledgements
We are grateful to Dr. Oili Kiikkilä and Prof. Heljä-Sisko Helmisaari for thoughtful comments, Dr. Joann von Weissenberg for checking the English language of this paper, to Anne Siika for making the figures and to previous members of the research group, particularly Dr. Laura Höijer and Dr. Outi Priha, for their contribution. The Finnish Forest Research Institute, Academy of Finland and Maj and Tor Nessling Foundation have funded several studies discussed in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Hinsinger.
Rights and permissions
About this article
Cite this article
Smolander, A., Kanerva, S., Adamczyk, B. et al. Nitrogen transformations in boreal forest soils—does composition of plant secondary compounds give any explanations?. Plant Soil 350, 1–26 (2012). https://doi.org/10.1007/s11104-011-0895-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0895-7