Abstract
Purpose
Tannin is the fourth most abundant biochemical compound in vascular plants. Due to its protein-binding capacity, tannin can interfere with soil nitrogen (N) biogeochemical cycling processes and potentially contribute to soil N conservation. Yet little is known about the effect and mechanism of tannin on regulating soil N transformation process.
Materials and methods
We devised microcosm study to evaluate the effects of different concentration and types of tannin on N-cycling processes. Soils were collected from subtropical Chinese fir plantation and incubated for 28 days. Two incubation experiments were carried out simultaneously; one was treated with different concentrations of condensed tannin (CT), including low (1 mg·g−1), middle (5 mg·g−1), and high (10 mg·g−1) concentrations of tannin, while the other was treated with different types of tannin including tannin acid (TA), catechin (CA), and tannin acid + catechin (TC). Soil enzyme activities, MBC, MBN, DOC, DON, soil N transformation rate and respiration were measured in both experiments.
Results and discussion
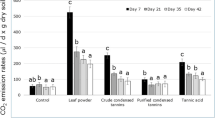
In both incubation experiments, tannin amendment significantly lowered soil nitrate content compared with control soil, as well as soil net nitrification and mineralization rates. In contrast, we observed consistent increase of soil enzyme activities involved in N cycling (amidase, N-acetyl-β-D-glocosaminidase, polyphenol oxidase, peroxidase, urease and laccase) under tannin treatments. This stimulating effect on soil enzyme activities is likely to due to the fact that tannin may serve as a carbon substrate for soil microorganisms rather than a toxic compound, as indicated by enhancing soil respiration with tannin addition. However, the stimulating effect on soil respiration eclipsed as the incubation proceeded. Furthermore, soil net mineralization rate, on average, decreased by 7.8%, 38.2%, and 69.1%, respectively, under low, middle, and high concentrations of tannin-added soils compared with control.
Conclusions
This study highlights the distinct role of tannin with different concentrations and types in regulating soil N cycling in a subtropical forest. Addition of tannin generally increased soil enzyme activities and soil respiration rate, whereas, the effects of different tannin type on soil N mineralization and nitrification remain largely unpredictable.






Similar content being viewed by others
Data availability
Data will be available upon request.
References
Adamczyk B, Adamczyk S, Smolander A, Kitunen V (2011) Tannic acid and Norway spruce condensed tannins can precipitate various organic nitrogen compounds. Soil Biol Biochem 43:628–637
Adamczyk S, Adamczyk B, Kitunen V, Smolander A (2015) Monoterpenes and higher terpenes may inhibit enzyme activities in boreal forest soil. Soil Biol Biochem 87:59–66
Adamczyk B, Kitunen V, Smolander A (2008) Protein precipitation by tannins in soil organic horizon and vegetation in relation to tree species. Biol Fert Soils 45:55–64
Adamczyk B, Kitunen V, Smolander A (2009) Polyphenol oxidase, tannase and proteolytic activity in relation to tannin concentration in the soil organic horizon under silver birch and Norway spruce. Soil Biol Biochem 41:2085–2093
Adamczyk B, Sietiö-M SP, Prommer J, Wild B, Hagner M, Pihlatie M, Fritze H, Richter A, Heinonsalo J (2019) Plant roots increase both decomposition and stable organic matter formation in boreal forest soil. Nat Commun 10(1):3982
Bradley RL, Titus BD, Preston CP (2000) Changes to mineral N cycling and microbial communities in black spruce humus after addition of (NH4)2SO4 and condensed tannins extracted from Klamia angustifolia and balsam fir. Soil Biol Biochem 32:1227–1240
Castells E, Peńuelas J, Valentine DW (2004) Are phenolic compounds released from the Mediterranean shrub Cistus albidus responsible for changes in N cycling in siliceous and calcareous soils? New Phytol 162(1):187–195
Chen C, Qiu H, Ma H, Imran S, Raza T, Gao R, Yin Y (2020) Response of the subtropical forest soil N transformations to tannin acid-organic nitrogen complexes. SN Appl Sci 2:1209
Clein JS, Schimel JP (1995) Nitrogen turnover and availability during succession from alder to poplar in Alaskan taiga forests. Soil Biol Biochem 27:743–752
Davis RI (1971) Relation of polyphenols to decomposition of organic matter and to pedogenic processes. Soil Sci 111:80–85
DeLuca TH, Nilsson MC, Zackrisson O (2002) Nitrogen mineralization and phenol accumulation along a fire chronosequence in northern Sweden. Oecologia 133:206–214
Di Y, Fu X, Wang H, Li W, Wang S (2018) N concentration of old leaves and twigs is more sensitive to stand density than that of young ones in Chinese fir plantations: a case study in subtropical China. J Forest Res 29:163–169
Dornbush ME (2007) Grasses, litter and their interaction affect microbial biomass and soil enzyme activity. Soil Biol Biochem 39:2241–2249
Feucht W, Treutter D (1999) The role of flavan-3-ols and proanthocyanidins in plant defense. In: Dakshini KMM, Foy CL (eds) Principles and practices in plant ecology – allochemical interactions. CRC Press, Roca Raton, pp 307–338
Fierer N, Schimel JP, Cates RG, Zou J (2001) Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biol Biochem 33:1827–1839
Geisseler D, Horwath WR, Joergensen RG, Ludwig B (2010) Pathways of nitrogen utilization by soil microorganisms- a review. Soil Biol Biochem 42:2058–2067
Gonzalez-Hernandez MP, Karchesy J, Starkey EE (2003) Research observation: hydrolysable and condensed tannins in plants of northwest Spain forests. J Range Manage 56:461–465
Hattenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15:238–242
Hernes PJ, Hedge JI (2000) Determination of condensed tannin monomers in environmental samples by capillary gas chromatography of acid depolymerization extracts. Anal Chem 72:5115–5124
Huang Z, Liao L, Wang S, Gao G (2000) Allelopathy of phenolics from decomposing stump-roots in replanted Chinese fir woodland. J Chem Ecol 26:2211–2219
Jenkinson DS (1988) Determination of microbial carbon and nitrogen in soil. In: Wilson J (ed) Advances in nitrogen cycling in agriculture ecosystems. CAB International, Wallingford, pp 368–385
Joanisse GD, Bradley RL, Preston CM, Munson AD (2007) Soil enzyme inhibition by condensed litter tannins may drive ecosystem structure and processes: the case of Kalmia angustifolia. New Phytol 175:535–546
Juntheikki MR, Julkunen-Titto R (2000) Inhibition of β-glucosidase and esterase by tannins from Betula, Salix, and Pinus species. J Chem Ecol 26:1151–1165
Kalburtji KL, Mosjidis JA, Mamolos AP (1999) Litter dynamics of low and high tannin sericea lespedeza plants under field conditions. Plant Soil 208:217–281
Kanerva S, Kitunen V, Kiikkil O, Loponene J, Smolander A (2006) Response of soil C and N transformations to tannin fractions originating from Scots pine and Norway spruce needles. Soil Biol Biochem 38(6):1364–1374
Kraus TEC, Dahlgren RA, Zasoski RJ (2003a) Tannins in nutrient dynamics of forest ecosystems-a review. Plant Soil 256:41–66
Kraus TEC, Yu Z, Preston CM, Dahlgren RA, Zasoski RJ (2003b) Linking chemical reactivity and protein precipitation to structural characteristics of foliar tannins. J Chem Ecol 29:703–730
Kraus TEC, Zasoski RJ, Dahlgren RA, Horwath WR, Preston CM (2004) Carbon and nitrogen dynamics in a forest soil amended with purified tannins from different plant species. Soil Biol Biochem 36:309–321
Ma H, Gao R, Yin Y, Yang Y (2016) Effects of leaf litter tannin on soil ammonium and nitrate content in two different forest soils of mount Wuyi, China. Toxicol Environ Chem 98:395–409
Nierop KGJ, Preston CM, Verstraten JM (2006) Linking the B ring hydroxylation pattern of condensed tannins to C, N and P mineralization. A case study using four tannins. Soil Biol Biochem 38:2794–2802
Parham J, Deng S (2000) Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol Biochem 32:1183–1190
Schimel JP, Cleve KV, Cate RG, Clausen TP, Reichardt PB (1996) Effects of balsam poplar (Populus balsamifera) tannins and low molecular weight phenolics on microbial activity in taiga floodplain soil: implications for changes in N cycling during succession. Can J Bot 74:84–90
Schweitzer JA, Marritch MD, Bailey JK, LeRoy CJ, Whitham TG (2008) From genes to ecosystems: the genetic basis of condensed tannins and their role in nutrient regulation in a populous model system. Ecosystems 11:1005–1020
Selmants PC, Schweitzer JA, Adair KL, Holeski LM, Whitham TG (2019) Genetic variation in tree leaf chemistry predicts the abundance and activity of autotrophic soil microorganisms. Ecosphere 10(8):e02795
State Forestry Administration of China (2014) Forestry resources report in China (2009–2013). China Forestry Publishing House, Beijing
Talbot JM, Finzi AC (2008) Differential effects of sugar maple, red oak, and hemlock tannins on carbon and nitrogen cycling in temperate forest soils. Oecologia 155:583–592
Triebasser DJ, Tharayil N, Preston CM, Gerard PD (2012) The susceptibility of soil enzymes to inhibition by leaf litter tannins is dependent on the tannin chemistry, enzyme class and vegetation history. New Phytol 196:1122–1132
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Yang Y, Wang L, Yang Z, Xu C, Xie J, Chen G, Lin C, Guo J, Liu X, Xiong D, Lin W, Chen S, He Z, Lin K, Jiang M, Lin T (2018) Large ecosystem service benefits of assisted natural regeneration. J Geophys Res Biogeosci 123(2):676–687
Zhang QF, Laanbroek HJ (2018) The effects of condensed tannins derived from senescing Rhizophora mangle leaves on carbon, nitrogen and phosphorus mineralization in a Distichlis spicata salt marsh soil. Plant Soil 433:37–53
Zhou LL, Shalom AD, Wu PF, Li SB, Jia YY, Ma XQ (2015) Litterfall production and nutrient return in different-aged Chinese fir (Cunninghamia lanceolata) plantations in South China. J For Res 26:79–89
Zong W, Wang J, He Y, Qiu Y, Guo D, Fu H (2018) Net nitrogen mineralization and enzyme activities in an alpine soil amended with litter tannins. J Plant Nutr Soil Sci 000:1–12
Acknowledgements
We are grateful to Chujun Tang for the assistance in the laboratory and field work and to Pengyu Jiao for the suggestion in statistical analysis.
Funding
Financial support was provided by a Science and Technology Department of Fujian Province (grant no. 2022J01576).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Yongfu Li
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Yao, Z., Cheng, L. et al. Condensed tannin addition decreased soil nitrate but increased soil enzyme activities in subtropical forest soil. J Soils Sediments 24, 537–551 (2024). https://doi.org/10.1007/s11368-023-03659-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03659-9