Abstract
The prepared activated zirconium oxide was characterized using different analytic techniques and investigated as a new inorganic sorbent to get rid of the chromium and barium ions from the waste stream. Several experiments have been performed, including the impact of contact time, pH, initial ion concentration, temperature, desorption, and the effect of interfering ions. Different isotherm kinetic models were investigated. The outcomes demonstrated that, the second-order kinetic model was appropriate, and the monolayer capacities for the chromium and barium ions were 35.9 and 33.9 mg/g, respectively. Finally, zirconium oxide was recommended to be used as a highly selective adsorbent for hazardous metal ions.
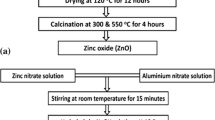
Graphical abstract












Similar content being viewed by others
References
Hassan HS, Abdel Moamen OA, Zaher AWF (2020) Neuro-Fuzzy inference system analysis on sorption studies of strontium and cesium cations onto a novel impregnated nano-zeolite. Adv Powd Techn 31:1125–1139
Khorasgani MD, Faghihian H, Givianrad MH, Azar PA, Tehrani MS (2022) Synthesis and application of a novel mesoporous SBA-15 sorbent functionalized by 2,4 dinitrophenyl hydrazine (DNPH) for simultaneous removal of Pb(II), Cr(III), Cd(II) and Co(II) from aqueous solutions: experimental design, kinetic, thermodynamic, and isotherm aspects. Adv Powd Techn 33:103201
Dakroury G, Abo-Zahra SF, Hassan HS, Ali HEA (2020) Improvement of the sorption behavior of aluminum silicate composite toward 134Cs and 60Co radionuclides by non-living biomass of Chlorella vulgaris. Environ Sci Pollu Res 27:21109–21125
Zhang J, Yan M, Sun G, Liu K (2021) Simultaneous removal of Cu(II), Cd(II), Cr(VI), and rhodamine B in wastewater using TiO2 nanofibers membrane loaded on porous fly ash ceramic support. Separ Purifi Technol 272:118888
Abdulkhair B, Salih M, Modwi A, Adam F, Elamin N, Seydou M, Rahali S (2021) Adsorption behavior of barium ions onto ZnO surfaces: experiments associated with DFT calculations. J Mol Struct 1223:128991
Majidnia Z, Idris A, Abd Majid M, Zin RM, Ponraj M (2015) Efficiency of barium removal from radioactive waste water using the combination of maghemite and titanium nanoparticles in PVA and alginate beads. Appl Radit Isotope 05:105–113
Fontana KB, Chaves ES, Kosera VS, Lenzi GG (2018) Barium removal by photo- catalytic process: an alternative for water treatment. J Water Process Eng 22:163–171
Kanwar VS, Sharma A, Srivastav AL, Rani L (2020) Phytoremediation of toxic metals present in soil and water environment: a critical review. Environ Sci Pollut Res 27:44835–44860
Prasad S, Yadav KK, Kumar S, Gupta N, Cabral-Pinto MMS, Rezania S, Radwan N, Alam J (2021) Chromium contamination and effect on environmental health and its remediation: a sustainable approaches. J Environ Manage 285:112174
Park JE, Shin JH, Oh W, Choi S-J, Kim J, Kim C, Jeon J (2022) Removal of hexavalent chromium(VI) from wastewater using chitosan-coated iron oxide nanocomposite membranes. Toxics 10(2):98. https://doi.org/10.3390/toxics10020098
Vital B, Bartacek J, Ortega-Bravo JC, Jeison D (2018) Treatment of acid mine drainage by forward osmosis: heavy metal rejection and reverse flux of draw solution constituents. Chem Eng J 332:85–91
Hargreaves AJ, Vale P, Whelan J, Alibardi L, Constantino C, Dotro G, Cartmell E, Campo P (2018) Impacts of coagulation-flocculation treatment on the size distribution and bioavailability of trace metals (Cu, Pb, Ni, Zn) in municipal wastewater. Water Res 128:120–128
Chen Q, Liang S, Zhang H, Liu D, Zhuo L (2021) Fabrication and characterization of W-Ni nanocomposites via a facile chemical co-precipitation route. Adv Powder Technol 32(3):908–915
Attallah MF, Hassan HS, Youssef MA (2019) Synthesis and sorption potential study of Al2O3-ZrO2-CeO2 composite material for removal of some radionuclides from radioactive waste effluent. Appl Radiat Isotop 147:40–47
Hassan HS, Elmaghraby EK (2019) Retention behavior of cesium radioisotope on poly (acrylamido-sulfonicacid) synthesized by chain polymerization. Appl Radiat Isot 146:40–47
Fawzy MA (2020) Biosorption of copper ions from aqueous solution by Codium Vermilara Optimization, kinetic, isotherm and thermodynamic studies. Adv Powder Technol 31:3724–3735
Li R, Zhai Z, Li Y, Yang T, Chen Y (2018) Kinetic study of heavy metals Cu and Zn removal during sewage sludge ash calcination in air and N2 atmospheres. J Hazard Mater 347:227–232
Sanad MMS, Farahat MM, Abdel Khalek MA (2021) One-step processing of low cost and superb natural magnetic adsorbent: kinetics and thermodynamics investigation for dye removal from textile wastewater. Adv Powd Technol 32:1573–1583
Shahrivar J, Gharabaghi M (2020) Separation of AuCN2-by activated carbon and functionalized graphene/activated carbon composite. Adv Powd Technol 31:4648–4656
Li J, Hui L, Zhang W, Lu J, Yang Y, Feng H (2021) Scalable production of ultra small TiO2 nano crystal/activated carbon composites by atomic layer deposition for efficient removal of organic pollutants. Adv Powd Technol 32:728–739
Dalmieda J, Kruse P (2019) Metal cation detection in drinking water. Sensors 19(3):5134
Hassan HS, Attia LA, Dakroury GA (2020) Exploration of the parameters affecting the radioactive europium removal from aqueous solutions by activated carbon-epoxy composite. Appl Radit Isotop 164:109278
Mahmoud ME, Saad EA, El-Khatib AM, Soliman MA, Allam EA (2017) Adsorptive removal of Zn(II), Co(II) and their radioactive isotopes 65Zn, 60Co on the surface of sodium nano bentonite coated with oleyl-amine. J Rad Nucl Appl 2(3):87–93
Dakroury GA, Abo-Zahra SF, Hassan HS, Fathy NA (2020) Utilization of silica–chitosan nanocomposite for removal of 152+154Eu radionuclide from aqueous solutions. J Radioanalyt Nucl Chem 323:439–455
K. Simeonidis K, Martinez-Boubeta C, Zamora-Perez P, Rivera-Gil P, Kaprara E, Kokkinos E, Mitrakas M (2018) Nanoparticles for heavy metal removal from drinking water. In: Environ Nanotech, Springer, 75–124.
Abdel Maksoud MIA, Sami NM, Hassan HS, Bekhit M, Ashour AH (2022) Novel adsorbent based on carbon-modified zirconia/spinel ferrite nanostructures: evaluation for the removal of cobalt and europium radionuclides from aqueous solutions. J Coll Interf Sci 607:111–124
Abdel Moamen OA, Hassan HS, El-Sherif EA (2017) Binary oxide composite adsorbent for copper, nickel and zinc cations removal from aqueous solutions. Desalin Water Treat 82:219–233
Liu X, Pang H, Liu X, Li Q, Zhang N, Mao L, Qiu M, Hu B, Yang H, Wang X (2021) Orderly porous covalent organic frameworks-based materials: superior adsorbents for pollutants removal from aqueous solutions. Innovation 2:100076
Dakroury GA, Ali SM, Hassan HS (2021) Assessment of adsorption performance of chitosan/ZrO2biosorbent composite towards Cs (I) and Co (II) metal ions from aqueous solution. J Polym Res 28:385
Zhang X, Zhang M, Zhang J, Zhang Q, Tsubaki N, Tan Y, Han Y (2019) Methane decomposition and carbon deposition over Ni/ZrO2 catalysts: comparison of amorphous, tetragonal, and monoclinic zirconia phase. Int J Hydrogen Energy 44(33):17887–17899
Aziz SB, Karim W, Brza M, Abdulwahid R, Raza SS, Al-Zangana S, Kadir M (2019) Ion transport study in CS: POZ based polymer membrane electrolytes using trukhan model. Int J Mol Sci 20(21):5265
Hassan HS, Madcour W, Elmaghraby EK (2019) Removal of radioactive cesium and europium from aqueous solutions using activated Al2O3 prepared by solution combustion. Mater Chem Phys 234:55–66
Chinchamalatpure VR, Chore SM, Patil SS, Chaudhari GN (2012) Synthesis and electrical characterization of ZrO2 Thin films on Si(100). J Mod Phys 3(1):69–73. https://doi.org/10.4236/jmp.2012.31010
Deng J, Lid S, Xiong L, Jiao Y, Yuan S, Wang J, Chen Y (2020) Preparation of nanostructured CeO2-ZrO2-based materials with stabilized surface area and their catalysis in soot oxidation. Appl Surf Sci 505:144301
Sharaf G, Hassan H (2014) Removal of copper ions from aqueous solution using silica derived from rice straw: comparison with activated charcoal. Int J Environ Sci Technol 11:1581–1590
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Dakroury GA, Abo-Zahra ShF, Hassan HS (2020) Utilization of olive pomace in nano MgO modification for sorption of Ni(II) and Cu(II) metal ions from aqueous solutions. Arab J Chem 13:6510–6522
Abdel Maksoud MIA, Sami NM, Hassan HS, Awed AS (2021) Sorption characteristics of bismuth tungstate nanostructure for removal of some radionuclides from aqueous solutions. Sep Purifi Technol 277:119478
Yang T, Wang Y, Sheng L, He C, Sun W, He Q (2020) Enhancing Cd(II) sorption by red mud with heat treatment: performance and mechanisms of sorption. J Environ Manag 255:109866
El-khalafawy A, Imam DM, Youssef MA (2022) Enhanced biosorption of europium and cesium ions from aqueous solution onto phalaris seed peel as environmental friendly biosorbent: equilibrium and kinetic studies. Appl Radiat Isotopes 190:110498
Panayotova MI (2001) Kinetics and thermodynamics of copper ions removal from wastewater by use of zeolite. Waste Manag 21:671–676
Bayramoglu G, Arica MY (2017) Polyethylenimine and tris(2-aminoethyl)amine modified p(GA–EGMA) microbeads for sorption of uranium ions: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 312(2):293–303. https://doi.org/10.1007/s10967-017-5216-z
Arica MY, Bayramoglu G (2016) Polyaniline coated magnetic carboxymethylcellulose beads for selective removal of uranium ions from aqueous solution. J Radioanal Nucl Chem 310(2):711–724. https://doi.org/10.1007/s10967-016-4828-z
Erkaya IA, Arica MY, Akbulut A, Bayramoglu G (2014) Biosorption of uranium(VI) by free and entrapped Chlamydomonas reinhardtii: kinetic, equilibrium and thermodynamic studies. J Radioanal Nucl Chem 299(3):1993–2003. https://doi.org/10.1007/s10967-014-2964-x
Youssef MA, Attia LA (2023) Novel nano Rosmarinus officinalis phytomass adsorbent for strontium and europium removal from aqueous solution: batch and packet techniques. J Radioanal Nucl Chem 332:1935–1952. https://doi.org/10.1007/s10967-023-08862-z
Ouadjenia-Marouf F, Marouf R, Schott J, Yahiaoui A (2013) Removal of Cu(II), Cd(II) and Cr(III) ions from aqueous solution by dam silt. Arab J Chem 11:401–406
Abdel Maksoud MIA, Murad GA, Zaher WF, Hassan HS (2023) Adsorption and separation of Cs(I) and Ba(II) from aqueous solution using zinc ferrite-humic acid nanocomposite. Sci Rep 13:5856
Jaegwan S, Jinwoo K, Yong-Gu L, Sangwon K, Changgil S (2021) Changes in adsorption mechanisms of radioactive barium, cobalt, and strontium ions using spent coffee waste biochars via alkaline chemical activation: enrichment effects of O-containing functional groups. Environ Res 199:111346
Kaveeshwar AR, Kumar PS, Revellame ED, Gang DD, Zappi ME, Subramaniam R (2018) Adsorption properties and mechanism of barium(II) and strontium(II) removal from fracking wastewater using pecan shell-based activated carbon. J Cleaner Prod 20:1–13
Hassan SSM, Kamel AH, Youssef MA, Aboterika AHA, Awwad NS (2020) Removal of barium and strontium from wastewater and radioactive wastes using a green bioadsorbent, Salvadora persica (Miswak). Desalin Water Treatm 192:306–314
Guan X (2022) Remediation and resource utilization of chromium(III)-containing tannery effluent based on chitosan-sodium alginate hydrogel. Carbohydr Polym 284:119179
Fei, Y, Deng, H, Wu, G, Luo, M, Chen, Y, Wang, X, Ye, H, Liu, T (2022) Insight into adsorption process and mechanisms of Cr(III) using carboxymethyl cellulosegpoly( acrylic acid-co-acrylamide)/attapulgite composite hydrogel. Environ Technol. 1–51
Alexis S, Ranjan R, Andrew G, Jason R, Marie J (2023) Selective adsorption of Cr(III) over Cr(VI) by starch-graft-itaconic acid hydrogels. J Hazard Mater Adv 10:100255
Abass MR, Youssef MA, Eid MA (2023) Inorganic composites based on carboxymethyl cellulose: preparation, characterization, sorption, and selectivity behavior for some radionuclides from radioactive solutions. Radiochim Acta. https://doi.org/10.1515/ract-2023-0214
Maleki F, Pacchioni G (2020) Characterization of acid and basic sites on zirconia surfaces and nanoparticles by adsorbed probe molecules: a theoretical study. Top Catal 63:1717–1730. https://doi.org/10.1007/s11244-020-01328-6
Nagaoka N, Yoshihara K, Feitosa V et al (2017) Chemical interaction mechanism of 10-MDP with zirconia. Sci Rep 7:45563. https://doi.org/10.1038/srep45563
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Maha A. Youssef (first author) was responsible for the measurement of the batch sorption study and the analysis of the data, writing, review, and editing. These measurements and their analyses were conducted in the Hot Laboratories and Waste Management Center- Egyptian Atomic Energy Authority. Abeer EL khalafawy (second author) contributed to the formal analysis, investigation, data curation, and review of the original draft. Hisham S. Hassan was responsible for conceptualization, supervision, visualization, and reviewing the original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The submitted manuscript is original and hasn’t been published elsewhere in any form or language.
Consent to publication
All authors agreed with the content and gave explicit consent to submit, and we obtained consent from the responsible authorities (the Egyptian atomic energy authority where the work has been carried out) before the work was submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Youssef, M.A., El-khalafawy, A. & Hassan, H.S. Insight into adsorption behavior of activated ZrO2 prepared by solution combustion for the removal of chromium and barium ions from aqueous solutions. J Radioanal Nucl Chem 333, 1883–1897 (2024). https://doi.org/10.1007/s10967-024-09388-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-024-09388-8