Abstract
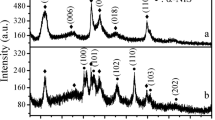
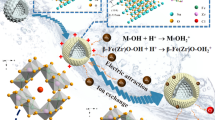
Hollow microspheres Bi2WO6 (HBWO) were prepared by the solvothermal method for the adsorption of U(VI) from aqueous solutions. Batch experiments and characterization analysis investigated the adsorption performance and mechanism. The results showed that the adsorption equilibrium could be reached in 30 min at T = 303 K, pH = 6, CU(VI) = 10 mg/L, m/V = 0.1 g/L. The removal efficiency of HBWO for U(VI) in solution was 95.53%, which was about 20% higher than Bi2WO6 nanosheets. The maximum Langmuir adsorption capacity could reach 523.13 mg/g. FTIR and XPS analysis indicated that the interaction mechanism of U(VI) with HBWO is mainly surface complexation of strong ionic bond (M–O) and hydroxyl (–OH) groups. In addition, HBWO has strong ion selectivity for U(VI) and good regeneration and reuse performance. These results demonstrated that HBWO might potentially remediate actual uranium-containing wastewater.














Similar content being viewed by others
References
Wang D, Song JA, Wen J, Yuan YH, Liu ZL, Lin S, Wang HY, Wang HL, Zhao SL, Zhao XM, Fang MH, Lei M, Li B, Wang N, Wang XL, Wu H (2018) Significantly enhanced uranium extraction from seawater with mass produced fully amidoximated nanofiber adsorbent. Adv Energy Mater 8:1802607. https://doi.org/10.1002/aenm.201802607
Han XW, Wang YQ, Cao XH, Dai Y, Liu YH, Dong ZM, Zhang ZB, Liu YH (2019) Adsorptive performance of ship-type nano-cage polyoxometalates for U(VI) in aqueous solution. Appl Surf Sci 484:1035–1040. https://doi.org/10.1016/j.apsusc.2019.04.121
Zou YD, Wang PY, Yao W, Wang XX, Liu YH, Yang DX, Wang LD, Hou J, Alsaedi A, Hayat T, Wang XK (2017) Synergistic immobilization of UO22+ by novel graphitic carbon nitride @ layered double hydroxide nanocomposites from wastewater. Chem Eng J 330:573–584. https://doi.org/10.1016/j.cej.2017.07.135
Merroun ML, Nedelkova M, Ojeda JJ, Reitz T, Fernandez ML, Arias JM, Romero-Gonzalez M, Selenska-Pobell S (2011) Bio-precipitation of uranium by two bacterial isolates recovered from extreme environments as estimated by potentiometric titration, TEM and X-ray absorption spectroscopic analyses. J Hazard Mater 197:1–10. https://doi.org/10.1016/j.jhazmat.2011.09.049
Yang H, Liu X, Hao M, Xie Y, Wang X, Tian H, Waterhouse GIN, Kruger PE, Telfer SG, Ma S (2021) Functionalized iron-nitrogen-carbon electrocatalyst provides a reversible electron transfer platform for efficient uranium extraction from seawater. Adv Mater 33:e2106621. https://doi.org/10.1002/adma.202106621
Heshmati H, Torab-Mostaedi M, Gilani HG, Heydari A (2015) Kinetic, isotherm, and thermodynamic investigations of uranium(VI) adsorption on synthesized ion-exchange chelating resin and prediction with an artificial neural network. Desalin Water Treat 55:1076–1087. https://doi.org/10.1080/19443994.2014.922495
Wang CH, Zhang J, Li J (2022) Easily synthesized mesoporous aluminum phosphate for the enhanced adsorption performance of U(VI) from aqueous solution. J Hazard Mater 432:128675. https://doi.org/10.1016/j.jhazmat.2022.128675
Dai Z, Zhen Y, Sun Y, Li L, Ding D (2021) ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium(VI) removal. Chem Eng J. https://doi.org/10.1016/j.cej.2021.129002
Dou W, Yang W, Zhao X, Pan Q (2019) Hollow cobalt sulfide for highly efficient uranium adsorption from aqueous solutions. Inorg Chem Front 6:3230–3236. https://doi.org/10.1039/c9qi00737g
Wei J, Zhang X, Liu Q, Li Z, Liu L, Wang J (2014) Magnetic separation of uranium by CoFe2O4 hollow spheres. Chem Eng J 241:228–234. https://doi.org/10.1016/j.cej.2013.12.035
Zhao J, Ge S, Liu L, Shao Q, Mai X, Zhao CX, Hao L, Wu T, Yu Z, Guo Z (2017) Microwave solvothermal fabrication of zirconia hollow microspheres with different morphologies using pollen templates and their dye adsorption removal. Ind Eng Chem Res 57:231–241. https://doi.org/10.1021/acs.iecr.7b04000
Li J-F, Chen Y, Wang Z, Liu Z-Q (2018) Self-templating synthesis of hollow copper tungstate spheres as adsorbents for dye removal. J Colloid Interface Sci 526:459–469. https://doi.org/10.1016/j.jcis.2018.05.023
Chen T, Liu LZ, Hu C, Huang HW (2021) Recent advances on Bi2WO6-based photocatalysts for environmental and energy applications. Chin J Catal 42:1413–1438. https://doi.org/10.1016/S1872-2067(20)63769-X
He Y, Wang D, Li X, Fu Q, Yin L, Yang Q, Chen H (2021) Photocatalytic degradation of tetracycline by metal-organic frameworks modified with Bi2WO6 nanosheet under direct sunlight. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.131386
Ye L, Deng Y, Wang L, Xie H, Su F (2019) Bismuth-based photocatalysts for solar photocatalytic carbon dioxide conversion. Chemsuschem 12:3671–3701. https://doi.org/10.1002/cssc.201901196
Krajnak A, Viglasova E, Galambos M, Krivosudsky L (2017) Application of HDTMA-intercalated bentonites in water waste treatment for U(VI) removal. J Radioanal Nucl Chem 314:2489–2499. https://doi.org/10.1007/s10967-017-5590-6
Kovalevskiy N, Cherepanova S, Gerasimov E, Lyulyukin M, Solovyeva M, Prosvirin I, Kozlov D, Selishchev D (2022) Enhanced photocatalytic activity and stability of Bi2WO6–TiO2-N nanocomposites in the oxidation of volatile pollutants. Nanomaterials (Basel). https://doi.org/10.3390/nano12030359
Chankhanittha T, Somaudon V, Photiwat T, Hemavibool K, Nanan S (2021) Preparation, characterization, and photocatalytic study of solvothermally grown CTAB-capped Bi2WO6 photocatalyst toward photodegradation of rhodamine B dye. Opt Mater. https://doi.org/10.1016/j.optmat.2021.111183
Yang Y, Cui J, Jin H, Cao F (2018) A three-dimensional (3D) structured Bi2WO6-palygorskite composite and their enhanced visible light photocatalytic property. Sep Purif Technol 205:130–139. https://doi.org/10.1016/j.seppur.2018.05.027
Yuan D, Chen L, Xiong X, Yuan L, Liao S, Wang Y (2016) Removal of uranium(VI) from aqueous solution by amidoxime functionalized superparamagnetic polymer microspheres prepared by a controlled radical polymerization in the presence of DPE. Chem Eng J 285:358–367. https://doi.org/10.1016/j.cej.2015.10.014
Xiong T, Jia L, Li Q, Zhang Y, Zhu W (2022) Highly efficient adsorptive extraction of uranium from wastewater by novel kaolin aerogel. Sci Total Environ 842:156916. https://doi.org/10.1016/j.scitotenv.2022.156916
Prabhu DR, Sengupta A, Murali MS, Pathak PN (2015) Role of diluents in the comparative extraction of Th(IV), U(VI) and other relevant metal ions by DHOA and TBP from nitric acid media and simulated wastes: reprocessing of U–Th based fuel in perspective. Hydrometallurgy 158:132–138. https://doi.org/10.1016/j.hydromet.2015.10.011
Khamirchi R, Hosseini-Bandegharaei A, Alahabadi A, Sivamani S, Rahmani-Sani A, Shahryari T, Anastopoulos I, Miri M, Hai Nguyen T (2018) Adsorption property of Br-PADAP-impregnated multiwall carbon nanotubes towards uranium and its performance in the selective separation and determination of uranium in different environmental samples. Ecotoxicol Environ Saf 150:136–143. https://doi.org/10.1016/j.ecoenv.2017.12.039
Wang C, Wang G, Xie S, Wang J, Guo Y (2022) Removal behavior and mechanisms of U(VI) in aqueous solution using aloe vera biochar with highly developed porous structure. J Radioanal Nucl Chem 331:2273–2283. https://doi.org/10.1007/s10967-022-08281-6
Li J, Wu Z, Duan Q, Li X, Li Y, Alsulami H, Alhodaly MS, Hayat T, Sun Y (2020) Simultaneous removal of U(VI) and Re(VII) by highly efficient functionalized ZIF-8 nanosheets adsorbent. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.122398
Zhong X, Liu Y, Liang W, Zhu Y, Hu B (2021) Construction of core-shell MOFs@COF hybrids as a platform for the removal of UO2(2+) and Eu(3+) ions from solution. ACS Appl Mater Interfaces 13:13883–13895. https://doi.org/10.1021/acsami.1c03151
Liao J, Ding L, Zhang Y, Zhu W (2022) Efficient removal of uranium from wastewater using pig manure biochar: understanding adsorption and binding mechanisms. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2021.127190
Zhang J, Zhang H, Liu Q, Song D, Li R, Liu P, Wang J (2019) Diaminomaleonitrile functionalized double-shelled hollow MIL-101 ( Cr) for selective removal of uranium from simulated seawater. Chem Eng J 368:951–958. https://doi.org/10.1016/j.cej.2019.02.096
Wu J, Zheng Z, Zhu K, Xiang C, Wang J, Liu J (2022) Adsorption performance and mechanism of g-C3N4/UiO-66 composite for U(VI) from aqueous solution. J Radioanal Nucl Chem 331:469–481. https://doi.org/10.1007/s10967-021-08116-w
Hu Y, Zhao C, Yin L, Wen T, Yang Y, Ai Y, Wang X (2018) Combining batch technique with theoretical calculation studies to analyze the highly efficient enrichment of U(VI) and Eu(III) on magnetic MnFe2O4 nanocubes. Chem Eng J 349:347–357. https://doi.org/10.1016/j.cej.2018.05.070
Zhang J, Wang D, Cao R, Li J (2022) Hollow Fe3O4 nanospheres covered by phosphate-modified layered double hydroxides for the removal of uranium(VI) from water and soil. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2022.120688
Rajaei A, Ghani K, Jafari M (2021) Modification of UiO-66 for removal of uranyl ion from aqueous solution by immobilization of tributyl phosphate. J Chem Sci. https://doi.org/10.1007/s12039-020-01864-4
Bai Z-Q, Yuan L-Y, Zhu L, Liu Z-R, Chu S-Q, Zheng L-R, Zhang J, Chai Z-F, Shi W-Q (2015) Introduction of amino groups into acid-resistant MOFs for enhanced U(VI) sorption. J Mater Chem A 3:525–534. https://doi.org/10.1039/c4ta04878d
Ahmad M, Yang K, Li L, Fan Y, Shah T, Zhang Q, Zhang B (2020) Modified tubular carbon nanofibers for adsorption of uranium(VI) from water. ACS Appl Nano Mater 3:6394–6405. https://doi.org/10.1021/acsanm.0c00837
Zhuang S, Cheng R, Kang M, Wang J (2018) Kinetic and equilibrium of U(VI) adsorption onto magnetic amidoxime-functionalized chitosan beads. J Clean Prod 188:655–661. https://doi.org/10.1016/j.jclepro.2018.04.047
Rabanimehr F, Farhadian M, Nazar ARS, Moghadam M (2021) Fabrication of Z-scheme Bi2WO6/CNT/TiO2 heterostructure with enhanced cephalexin photodegradation: optimization and reaction mechanism. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.116728
Krishnan SAG, Abinaya S, Arthanareeswaran G, Govindaraju S, Yun K (2022) Surface-constructing of visible-light Bi2WO6/CeO2 nanophotocatalyst grafted PVDF membrane for degradation of tetracycline and humic acid. J Hazard Mater 421:126747. https://doi.org/10.1016/j.jhazmat.2021.126747
Liao J, Xiong T, Ding L, Xie Y, Zhang Y, Zhu W (2022) Design of a renewable hydroxyapatite-biocarbon composite for the removal of uranium(VI) with high-efficiency adsorption performance. Biochar. https://doi.org/10.1007/s42773-022-00154-1
Chen L, Zhao D, Chen S, Wang X, Chen C (2016) One-step fabrication of amino functionalized magnetic graphene oxide composite for uranium(VI) removal. J Colloid Interface Sci 472:99–107. https://doi.org/10.1016/j.jcis.2016.03.044
Wang Y, Lin Z, Zhu J, Liu J, Yu J, Liu Q, Chen R, Li Y, Wang J (2022) Co-construction of molecular-level uranyl-specific “nano-holes” with amidoxime and amino groups on natural bamboo strips for specifically capturing uranium from seawater. J Hazard Mater 437:129407. https://doi.org/10.1016/j.jhazmat.2022.129407
He Y, Gao H, Liu J (2022) A visible-light-active CuS/MoS(2)/Bi(2)WO(6) aptamer sensitively detects the non-steroidal anti-inflammatory drug diclofenac. Nanomaterials (Basel). https://doi.org/10.3390/nano12162834
Acknowledgements
This work was supported by the National Natural Science Foundation of China (42177074) and the Open Funding for Innovation Platform of Education Department in Hunan Province (22C0233).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, Z., Liu, J., Yu, H. et al. Adsorption performance and mechanism of U(VI) in aqueous solution by hollow microspheres Bi2WO6. J Radioanal Nucl Chem 332, 1755–1765 (2023). https://doi.org/10.1007/s10967-023-08842-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08842-3