Abstract
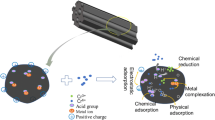
Herein, a novel aloe vera biochar (PBC) with highly developed porous structure was prepared to remove U(VI) in aqueous solution. The specific surface area of PBC was 577.3 m2/g, which was 100 times that of unmodified biochar. It had good environmental adaptability because of its fast removal rate, excellent adsorption performance in a wide pH range and selective adsorption capacity. And it could be recycled. The mechanism of the U(VI) adsorption by PBC was the surface complexation, in which the phosphate groups played a major role. Therefore, the development of PBC expanded the potential application of aloe vera wastes.
Graphical abstract






Similar content being viewed by others
References
Su M, Tsang DCW, Ren X, Shi Q, Tang J, Zhang H, Kong L, Hou L, Song G, Chen D (2019) Removal of U(VI) from nuclear mining effluent by porous hydroxyapatite: evaluation on characteristics, mechanisms and performance. Environ Pollut 254(Pt A):112891. https://doi.org/10.1016/j.envpol.2019.07.059
Jin J, Li S, Peng X, Liu W, Zhang C, Yang Y, Han L, Du Z, Sun K, Wang X (2018) HNO3 modified biochars for uranium (VI) removal from aqueous solution. Bioresour Technol 256:247–253. https://doi.org/10.1016/j.biortech.2018.02.022
Aljarrah MT, Al-Harahsheh MS, Alrebaki MA, Mayyas M (2020) Concentrative isolation of uranium traces in aqueous solutions via resurfaced-magnetic carbon nanotube suspension. J Environ Manage 271:110970. https://doi.org/10.1016/j.jenvman.2020.110970
Zhu J, Liu Q, Li Z, Liu J, Zhang H, Li R, Wang J (2018) Efficient extraction of uranium from aqueous solution using an amino-functionalized magnetic titanate nanotubes. J Hazard Mater 353:9–17. https://doi.org/10.1016/j.jhazmat.2018.03.042
Yang P, Zhang H, Liu Q, Liu J, Chen R, Yu J, Hou J, Bai X, Wang J (2019) Nano-sized architectural design of multi-activity graphene oxide (GO) by chemical post-decoration for efficient uranium(VI) extraction. J Hazard Mater 375:320–329. https://doi.org/10.1016/j.jhazmat.2019.05.005
Zhao C, Liu J, Deng Y, Tian Y, Zhang G, Liao J, Yang J, Yang Y, Liu N, Sun Q (2019) Uranium (VI) adsorption from aqueous solutions by microorganism-graphene oxide composites via an immobilization approach. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.117624
Zhang Q, Wang Y, Wang Z, Zhang Z, Wang X, Yang Z (2021) Active biochar support nano zero-valent iron for efficient removal of U(VI) from sewage water. J Alloy Compd. https://doi.org/10.1016/j.jallcom.2020.156993
Li L, Yang M, Lu Q, Zhu W, Ma H, Dai L (2019) Oxygen-rich biochar from torrefaction: a versatile adsorbent for water pollution control. Bioresour Technol 294:122142. https://doi.org/10.1016/j.biortech.2019.122142
Wei D, Li B, Huang H, Luo L, Zhang J, Yang Y, Guo J, Tang L, Zeng G, Zhou Y (2018) Biochar-based functional materials in the purification of agricultural wastewater: fabrication, application and future research needs. Chemosphere 197:165–180. https://doi.org/10.1016/j.chemosphere.2017.12.193
Giannakoudakis DA, Hosseini-Bandegharaei A, Tsafrakidou P, Triantafyllidis KS, Kornaros M, Anastopoulos I (2018) Aloe vera waste biomass-based adsorbents for the removal of aquatic pollutants: a review. J Environ Manag 227:354–364. https://doi.org/10.1016/j.jenvman.2018.08.064
Guilhen SN, Mašek O, Ortiz N, Izidoro JC, Fungaro DA (2019) Pyrolytic temperature evaluation of macauba biochar for uranium adsorption from aqueous solutions. Biomass Bioenerg 122:381–390. https://doi.org/10.1016/j.biombioe.2019.01.008
Zhao L, Zheng W, Masek O, Chen X, Gu B, Sharma BK, Cao X (2017) Roles of phosphoric acid in biochar formation: synchronously improving carbon retention and sorption capacity. J Environ Qual 46(2):393–401. https://doi.org/10.2134/jeq2016.09.0344
Chu G, Zhao J, Huang Y, Zhou D, Liu Y, Wu M, Peng H, Zhao Q, Pan B, Steinberg CEW (2018) Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ Pollut 240:1–9. https://doi.org/10.1016/j.envpol.2018.04.003
Shao D, Li Y, Wang X, Hu S, Wen J, Xiong J, Asiri AM, Marwani HM (2017) Phosphate-functionalized polyethylene with high adsorption of uranium(VI). ACS Omega 2(7):3267–3275. https://doi.org/10.1021/acsomega.7b00375
Li L, Ma R, Wen T, Gu P, Zhang S, Zheng M, Wu X, Zhang X, Hayat T, Wang X (2019) Functionalization of carbon nanomaterials by means of phytic acid for uranium enrichment. Sci Total Environ 694:133697. https://doi.org/10.1016/j.scitotenv.2019.133697
Ma D, Hu S, Li Y, Xu Z (2019) Adsorption of uranium on phosphoric acid-activated peanut shells. Sep Sci Technol 55(9):1623–1635. https://doi.org/10.1080/01496395.2019.1606016
Pan N, Jin Y, Wang X, Hu X, Chi F, Zou H, Xia C (2018) A Self-assembled supramolecular material containing phosphoric acid for ultrafast and efficient capture of uranium from acidic solutions. ACS Sustain Chem Eng 7(1):950–960. https://doi.org/10.1021/acssuschemeng.8b04596
Liu X, Li J, Wang X, Chen C, Wang X (2015) High performance of phosphate-functionalized graphene oxide for the selective adsorption of U(VI) from acidic solution. J Nucl Mater 466:56–64. https://doi.org/10.1016/j.jnucmat.2015.07.027
Hu R, Xiao J, Wang T, Chen G, Chen L, Tian X (2020) Engineering of phosphate-functionalized biochars with highly developed surface area and porosity for efficient and selective extraction of uranium. Chem Eng J. https://doi.org/10.1016/j.cej.2019.122388
Dai L, Li L, Zhu W, Ma H, Huang H, Lu Q, Yang M, Ran Y (2020) Post-engineering of biochar via thermal air treatment for highly efficient promotion of uranium(VI) adsorption. Bioresour Technol 298:122576. https://doi.org/10.1016/j.biortech.2019.122576
Ahmed MB, Zhou JL, Ngo HH, Guo W, Chen M (2016) Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour Technol 214:836–851. https://doi.org/10.1016/j.biortech.2016.05.057
Xia Y, Yang T, Zhu N, Li D, Chen Z, Lang Q, Liu Z, Jiao W (2019) Enhanced adsorption of Pb(II) onto modified hydrochar: modeling and mechanism analysis. Bioresour Technol 288:121593. https://doi.org/10.1016/j.biortech.2019.121593
Li H, Hu J, Yao L, Shen Q, An L, Wang X (2020) Ultrahigh adsorbability towards different antibiotic residues on fore-modified self-functionalized biochar: competitive adsorption and mechanism studies. J Hazard Mater 390:122127. https://doi.org/10.1016/j.jhazmat.2020.122127
Turk Sekulic M, Boskovic N, Slavkovic A, Garunovic J, Kolakovic S, Pap S (2019) Surface functionalised adsorbent for emerging pharmaceutical removal: adsorption performance and mechanisms. Process Saf Environ Prot 125:50–63. https://doi.org/10.1016/j.psep.2019.03.007
Peng H, Gao P, Chu G, Pan B, Peng J, Xing B (2017) Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ Pollut 229:846–853. https://doi.org/10.1016/j.envpol.2017.07.004
Puziy AM, Poddubnaya OI, Socha RP, Gurgul J, Wisniewski M (2008) XPS and NMR studies of phosphoric acid activated carbons. Carbon 46(15):2113–2123. https://doi.org/10.1016/j.carbon.2008.09.010
Uchimiya M, Hiradate S (2014) Pyrolysis temperature-dependent changes in dissolved phosphorus speciation of plant and manure biochars. J Agric Food Chem 62(8):1802–1809. https://doi.org/10.1021/jf4053385
Deng Y, Huang S, Dong C, Meng Z, Wang X (2020) Competitive adsorption behaviour and mechanisms of cadmium, nickel and ammonium from aqueous solution by fresh and ageing rice straw biochars. Bioresour Technol 303:122853. https://doi.org/10.1016/j.biortech.2020.122853
Shan R, Shi Y, Gu J, Wang Y, Yuan H (2020) Single and competitive adsorption affinity of heavy metals toward peanut shell-derived biochar and its mechanisms in aqueous systems. Chin J Chem Eng 28(5):1375–1383. https://doi.org/10.1016/j.cjche.2020.02.012
Zhang D, Wang T, Zhi J, Zheng Q, Chen Q, Zhang C, Li Y (2020) Utilization of jujube biomass to prepare biochar by pyrolysis and activation: characterization, adsorption characteristics, and mechanisms for nitrogen. Materials (Basel). https://doi.org/10.3390/ma13245594
Kim WK, Shim T, Kim YS, Hyun S, Ryu C, Park YK, Jung J (2013) Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. Bioresour Technol 138:266–270. https://doi.org/10.1016/j.biortech.2013.03.186
Kuo H-C, Lin Y-G, Chiang C-L, Liu S-H (2021) FeN@N-doped graphitic biochars derived from hydrothermal-microwave pyrolysis of cellulose biomass for fuel cell catalysts. J Anal Appl Pyrol. https://doi.org/10.1016/j.jaap.2020.104991
Lam SS, Yek PNY, Ok YS, Chong CC, Liew RK, Tsang DCW, Park YK, Liu Z, Wong CS, Peng W (2020) Engineering pyrolysis biochar via single-step microwave steam activation for hazardous landfill leachate treatment. J Hazard Mater 390:121649. https://doi.org/10.1016/j.jhazmat.2019.121649
Liu L, Li Y, Fan S (2019) Preparation of KOH and H3PO4 modified biochar and its application in methylene blue removal from aqueous solution. Processes. https://doi.org/10.3390/pr7120891
Huang Y, Li S, Lin H, Chen J (2014) Fabrication and characterization of mesoporous activated carbon from Lemna minor using one-step H3PO4 activation for Pb(II) removal. Appl Surf Sci 317:422–431. https://doi.org/10.1016/j.apsusc.2014.08.152
Huang S, Pang H, Li L, Jiang S, Wen T, Zhuang L, Hu B, Wang X (2018) Unexpected ultrafast and high adsorption of U(VI) and Eu(III) from solution using porous Al2O3 microspheres derived from MIL-53. Chem Eng J 353:157–166. https://doi.org/10.1016/j.cej.2018.07.129
Ying D, Hong P, Jiali F, Qinqin T, Yuhui L, Youqun W, Zhibin Z, Xiaohong C, Yunhai L (2020) Removal of uranium using MnO2/orange peel biochar composite prepared by activation and in-situ deposit in a single step. Biomass Bioenerg. https://doi.org/10.1016/j.biombioe.2020.105772
Han B, Zhang E, Cheng G, Zhang L, Wang D, Wang X (2018) Hydrothermal carbon superstructures enriched with carboxyl groups for highly efficient uranium removal. Chem Eng J 338:734–744. https://doi.org/10.1016/j.cej.2018.01.089
Li X, Pan H, Yu M, Wakeel M, Luo J, Alharbi NS, Liao Q, Liu J (2018) Macroscopic and molecular investigations of immobilization mechanism of uranium on biochar: EXAFS spectroscopy and static batch. J Mol Liq 269:64–71. https://doi.org/10.1016/j.molliq.2018.08.039
Liao J, Zhang Y (2020) Effective removal of uranium from aqueous solution by using novel sustainable porous Al2O3 materials derived from different precursors of aluminum. Inorg Chem Front 7(3):765–776. https://doi.org/10.1039/c9qi01426h
El-sherif RM, Lasheen TA, Jebril EA (2017) Fabrication and characterization of CeO 2 -TiO 2 -Fe 2 O 3 magnetic nanoparticles for rapid removal of uranium ions from industrial waste solutions. J Mol Liq 241:260–269. https://doi.org/10.1016/j.molliq.2017.05.119
Liu L, Lin X, Li M, Chu H, Wang H, Xie Y, Du Z, Liu M, Liang L, Gong H, Zhou J, Li Z, Luo X (2020) Microwave-assisted hydrothermal synthesis of carbon doped with phosphorus for uranium(VI) adsorption. J Radioanal Nucl Chem 327(1):73–89. https://doi.org/10.1007/s10967-020-07453-6
Li M, Liu H, Chen T, Dong C, Sun Y (2019) Synthesis of magnetic biochar composites for enhanced uranium(VI) adsorption. Sci Total Environ 651(Pt 1):1020–1028. https://doi.org/10.1016/j.scitotenv.2018.09.259
Zhang Y, Ye T, Wang Y, Zhou L, Liu Z (2021) Adsorption of uranium(VI) from aqueous solution by phosphorylated luffa rattan activated carbon. J Radioanal Nucl Chem 327(3):1267–1275. https://doi.org/10.1007/s10967-020-07592-w
Zhou Y, Xiao J, Hu R, Wang T, Shao X, Chen G, Chen L, Tian X (2020) Engineered phosphorous-functionalized biochar with enhanced porosity using phytic acid-assisted ball milling for efficient and selective uptake of aquatic uranium. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.112659
Albayari M, Nazal MK, Khalili FI, Nordin N, Adnan R (2021) Biochar derived from Salvadora persica branches biomass as low-cost adsorbent for removal of uranium(VI) and thorium(IV) from water. J Radioanal Nucl Chem 328(2):669–678. https://doi.org/10.1007/s10967-021-07667-2
Alahabadi A, Singh P, Raizada P, Anastopoulos I, Sivamani S, Dotto GL, Landarani M, Ivanets A, Kyzas GZ, Hosseini-Bandegharaei A (2020) Activated carbon from wood wastes for the removal of uranium and thorium ions through modification with mineral acid. Colloids Surf A. https://doi.org/10.1016/j.colsurfa.2020.125516
Tao Y, Han S, Zhang Q, Yang Y, Shi H, Akindolie MS, Jiao Y, Qu J, Jiang Z, Han W, Zhang Y (2020) Application of biochar with functional microorganisms for enhanced atrazine removal and phosphorus utilization. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.120535
Chen Y, Ning P, Miao R, He L, Guan Q (2021) Resource utilization of agricultural residues: one-step preparation of biochar derived from Pennisetum giganteum for efficiently removing chromium from water in a wide pH range. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-021-15388-y
Deng J, Li X, Liu Y, Zeng G, Liang J, Song B, Wei X (2018) Alginate-modified biochar derived from Ca(II)-impregnated biomass: Excellent anti-interference ability for Pb(II) removal. Ecotoxicol Environ Saf 165:211–218. https://doi.org/10.1016/j.ecoenv.2018.09.013
Zhang X, Lin X, He Y, Luo X (2019) Phenolic hydroxyl derived copper alginate microspheres as superior adsorbent for effective adsorption of tetracycline. Int J Biol Macromol 136:445–459. https://doi.org/10.1016/j.ijbiomac.2019.05.165
Liu S, Li J, Xu S, Wang M, Zhang Y, Xue X (2019) A modified method for enhancing adsorption capability of banana pseudostem biochar towards methylene blue at low temperature. Bioresour Technol 282:48–55. https://doi.org/10.1016/j.biortech.2019.02.092
Chen Y, Li M, Li Y, Liu Y, Chen Y, Li H, Li L, Xu F, Jiang H, Chen L (2021) Hydroxyapatite modified sludge-based biochar for the adsorption of Cu(2+) and Cd(2+): adsorption behavior and mechanisms. Bioresour Technol 321:124413. https://doi.org/10.1016/j.biortech.2020.124413
Pap S, Taggart MA, Shearer L, Li Y, Radovic S, Turk Sekulic M (2021) Removal behaviour of NSAIDs from wastewater using a P-functionalised microporous carbon. Chemosphere 264(Pt 1):128439. https://doi.org/10.1016/j.chemosphere.2020.128439
Tian Y, Liu L, Ma F, Zhu X, Dong H, Zhang C, Zhao F (2021) Synthesis of phosphorylated hyper-cross-linked polymers and their efficient uranium adsorption in water. J Hazard Mater 419:126538. https://doi.org/10.1016/j.jhazmat.2021.126538
Nie X, Jiang Y, Dong F, Cheng W, Wang J, Ding C, Liu M, Zhang Y, Xia X (2021) Amide and phosphate groups modified bifunctional luffa fiber for highly efficient removal of U(VI) from real uranium wastewater. J Radioanal Nucl Chem 328(2):591–604. https://doi.org/10.1007/s10967-021-07670-7
Ahmed W, Nunez-Delgado A, Mehmood S, Ali S, Qaswar M, Shakoor A, Chen DY (2021) Highly efficient uranium (VI) capture from aqueous solution by means of a hydroxyapatite-biochar nanocomposite: Adsorption behavior and mechanism. Environ Res 201:111518. https://doi.org/10.1016/j.envres.2021.111518
Funding
This work was supported in part by the Project supported by the Natural Science Foundation of Hunan Province, China (No. 2020JJ30579, No. 2020JJ5492); the National Natural Science Foundation of China (No. 51904155).
Author information
Authors and Affiliations
Contributions
Conceptualization: CW, GW and SX; Methodology: CW, GW and SX; Formal analysis and investigation: CW; Writing -original draft preparation: CW; Funding acquisition: SX and GW; Resources: SX and GW; Supervision: JW and YG; Writing—review and editing: CW, SX, GW, YG and JW.
Corresponding author
Ethics declarations
Conflict of interests
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, C., Wang, G., Xie, S. et al. Removal behavior and mechanisms of U(VI) in aqueous solution using aloe vera biochar with highly developed porous structure. J Radioanal Nucl Chem 331, 2273–2283 (2022). https://doi.org/10.1007/s10967-022-08281-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08281-6