Abstract
Background & aims: Ascites is a frequent complication of cirrhosis. In intensive care units, initial hemodynamic assessment is frequently performed by echocardiography. This study evaluated the feasibility and usefulness of early hemodynamic assessment in the gastroenterology ward. Methods: This observational cohort study prospectively included all patients admitted to a teaching hospital’s gastroenterology unit for decompensated cirrhosis. A gastroenterologist with minimal training and an intensivist both performed an echocardiography exam. The primary outcome was inter-rater agreement and reliability for three echocardiography parameters: visual LVEF (Left Ventricular Ejection Fraction), subaortic VTI (velocity time integral) and E wave velocity. Secondary outcomes were agreement for presence of pleural effusion, description of 3 hemodynamics profiles (hypovolemic, hyperkinetic and intermediate), and 28-day mortality.Results: From March 2018 to March 2020, 53 patients were included. The median age was 62 years and 81% were men. Patients presented mostly advanced liver disease, with 43% Child-Pugh C and median MELD score of 15.2. The limits of agreement between intensivists and gastroenterologists for subaortic VTI were − 6.6 to 7.2 cm, and ranged from − 0.6 to 0.37 m.s-1 for E wave velocity. Clinically significant differences between intensivists and gastroenterologists were found in 22% for subaortic VTI and 24.5% for E wave velocity. Reliability was good for subaortic VTI (ICC: 0.79, 95% CI [0.58; 0.9;]) and moderate for E wave velocity (0.53, 95% CI [0.19; 0.74]). The three hemodynamics profiles had different prognosis, with a 28-day mortality for Hypovolemic, Intermediate and Hyperkinetic group of 31, 18, and 4%, respectively.Conclusion: Reliability of hemodynamic assessment by gastroenterologists was good, while agreement was unsatisfactory, advocating for further training. Transthoracic echocardiography can differentiate hypovolemia from hyperkinetic states. The role of transthoracic echocardiography in managing decompensated cirrhosis requires further study.
Clinical trial number: NCT03650660.
Lay Summary
-
Echocardiographic hemodynamic evaluation can be performed by gastroenterologists after a short training.
-
Transthoracic echocardiography evaluation by intensivists defined three hemodynamic profiles in patients with decompensated cirrhosis: Hypovolemic, Hyperkinetic and intermediate, with distinct prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
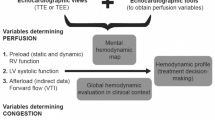
Decompensated cirrhosis is associated with high mortality, especially in case of refractory ascite. Decompensated cirrhosis with ascite impairs quality of life, increases the total number of hospitalizations. This lead to increased use of chronic medication [1], leads to spontaneous bacterial peritonitis, hepato-renal syndrom, septic shock and subsequent increased mortality [2][3]. It is now widely admitted that cardiopulmonary hemodynamics is predictive of relevant outcomes, both in patients with compensated and decompensated cirrhosis [4].
Several hemodynamic variations occur during cirrhosis, resulting in systemic, splanchnic vasodilation and capillary leak, subsequently leading to ascites, edema and hypovolemia [5]. To date, there is no recommendation for a systematic hemodynamic assessment in the initial phase of decompensated cirrhosis for patients hospitalized on gastroenterology wards. For patients on general wards, hemodynamic assessment needs to be non-invasive. Even in the ICU, where invasive hemodynamic monitoring is usually performed, transthoracic echocardiography (TTE) is widely used as a non-invasive tool for initial hemodynamic assessment [6, 7]. As evaluation of ascites and pleural effusion is also important, TTE can be coupled with pleural and ascites assessment as a POCUS (point of care ultrasound) assessment [8]. Several studies have shown that providing a short training in echocardiography to residents without previous knowledge in echography appears feasible and efficient to allow a simple and reliable assessment of cardiovascular structure [9, 10] and hemodynamic status [11, 12].
The primary aim of our study was to evaluate the agreement and reliability of the TTE assessment performed in the initial phase of the decompensated cirrhosis by expert intensivists and non-expert gastroenterologists after a short training. The secondary objectives consisted in (1) evaluating the agreement and reliability between gastroenterologist and intensivist for pleural effusion, (2) describing hemodynamic profiles in decompensated cirrhosis with edema and ascites and (3) correlating hemodynamic profiles with 28-day mortality.
1.1 Patients & methods
The EchoCirrho study was a prospective, observational, pilot study conducted in the gastroenterology department of Nîmes University Hospital (France) from March 30, 2018, to March 1, 2020. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local ethics committee (Personal Protection Committee - Ile de France VI n° 89 − 17, on 02/21/2018). The study was prospectively registered at clinicaltrials.gov (NCT03650660). Written informed consent was obtained from each patient included in the study.
1.2 Patients
Patients were enrolled in the present study if they met the following inclusion criteria: adult patients admitted to the gastroenterology ward for known or suspected decompensated cirrhosis diagnosed by biopsy or based on usual clinical/biological/radiological criteria. Decompensated cirrhosis was defined as cirrhosis associated with edema of the lower limbs, and/or ascites.
Exclusion criteria were: patients already enrolled in the present study, patients with hemodynamic instability related to gastrointestinal bleeding, septic shock or decompensated cardiac disease (severe cardiac failure, massive acute pulmonary edema, endocarditis, severe valvulopathy, cardiac tamponade, intracardiac thrombus) in order not to delay therapeutic management and ICU or cardiology ward transfer, under guardianship, pregnant or breastfeeding woman and poor echogenicity.
1.3 Outcome measures
The primary outcome was agreement and reliability of the TTE assessment performed in decompensated cirrhosis patients by intensivists versus gastroenterologists for quantification of Left Ventricular Ejection Fraction (LVEF), mitral E wave velocity, and subaortic velocity time integral (VTI). The agreement and reliability between intensivists versus gastroenterologists for presence of pleural effusion was a secondary outcome measure. Other secondary outcomes were to describe hemodynamic profiles using the data generated by experts only and to correlate these hemodynamic profiles with 28-day mortality.
1.4 Data
The following clinical and demographic data were collected at admission: age, sex, etiology of cirrhosis, severity of cirrhosis evaluated by the Child-Pugh [13] and MELD score [14], comorbidities, treatment that could influence cardiovascular status (beta blocker, diuretics, antihypertensive agents), and clinical examination.
The following biological data were collected at inclusion: prothrombin ratio (PR), International Normalized Ratio (INR), platelet count, hemoglobin level, C-reactive protein level, and serum levels of alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), bilirubin, creatinine, urea, sodium, potassium, albumin, gamma glutamyl transferase (GGT), and alkaline phosphatase (ALP).
At day 28, all treatments received during hospitalization in the gastroenterology ward and vital status were recorded. The REDCap® (Vanderbilt University, Nashville, United States of America) software was used to record and secure data collection [15].
1.5 Echocardiography data
We performed two independent POCUS exams for each patient on the same day; one by a gastroenterologist considered as a non-expert first and subsequently performed by an intensivist considered as an expert in echocardiography in the next hour. Echocardiographic assessments were performed immediately after admission in gastroenterology ward and before starting any treatment. Ultrasound exams were performed with a MEDISON M70 model (Samsung) device using a cardiac US probe PE 2–4 Hz (Samsung, Seoul, Korea).
Gastroenterology physicians participating in the study underwent a training protocol combining 2 h of training course and 2 days of hands-on training in an intensive care unit, supervised by an intensivist.
All intensivists were well-trained physicians (level 2 or 3, [16]) with more than 5 years of practice and a national diploma in echocardiography.
All echocardiographic measurements were performed according to international guidelines for TTE assessment [17].
-
Data collected by non-expert operators were:
-
a semi quantitative (≥ 40% or < 40%) visual LVEF assessment obtained from an apical 2 and 4 chamber view.
-
mitral E wave velocity (m.s-1) obtained from an apical 4 chamber view.
-
subaortic VTI (cm) obtained from an apical 5 chamber view.
-
qualitative assessment of pleural effusion.
-
-
Data collected by experts only were:
-
a semi quantitative (≥ 40% or < 40%) visual LVEF assessment obtained from an apical 2 and 4 chamber view. Several studies performed both in cardiology or ICU patients have demonstrated that eyeballing (visual) semi quantitative LVEF (normal, moderate or severe impairment) assessment is valuable, especially in acute conditions[18,19,20,21].
-
mitral E wave velocity (m.s-1) and mitral A wave velocity (m.s-1) obtained from an apical 4 chamber view.
-
subaortic VTI (cm) obtained from an apical 5 chamber view.
-
mitral S wave velocity obtained from tissue Doppler imaging from an apical 5 chambers view.
-
Ea wave velocity (cm.s-1) velocity obtained from tissue Doppler imaging from an apical 5 chambers view.
-
inferior vena cava collapsibility index (%) obtained from a subcostal view [22].
-
the presence of intrapulmonary shunt, the presence of a valvulopathy, left atrium surface area (cm2), left ventricle diameter (cm), septal wall thickness (mm), posterior wall thickness (mm).
-
tricuspid annular plane systolic excursion (TAPSE) (mm), tricuspid annular plane systolic velocity (TAPSV) (cm.s-1), the presence of cardiac chamber dilation, pulmonary arterial hypertension and pericardial effusion.
-
1.6 Assessment of pleural effusion by Lung ultrasound and ascites assessment
This was done with the same probe than for cardiac assessment. For pleural effusion, an eight-spots lung ultrasound exam was performed in order to diagnose absence or presence of pleural effusion, which is frequent in decompensated cirrhosis patients. According to international guidelines [23], we performed four-spots exam on both right and left thoracic area: 2 anterior spots and 2 lateral spots on the axillary line (one basal and one upward). Pleural effusion appears as a non-echoic area around a consolidated lung area. The diagnostic accuracy of ultrasonography for the diagnosis of pleural effusion has been shown to be significantly better than clinical assessment or conventional chest X-Ray [24, 25].
For ascites, as recommended for ICU patients for determining the best location for ascites paracentesis [26], we performed a five spots exam: peri hepatic, peri splenic, Douglas space and left and right hypogastric areas.
1.7 Hemodynamic profiles
Recent studies have described several hemodynamic phenotypes (profiles) during shock, especially hypovolemic and hyperkinetic (vasoplegic) profiles. These phenotypes are based on the combination of subaortic VTI, mitral E wave velocity and LVEF values [27]:
-
The hyperkinetic profile was defined as the combination of subaortic VTI > 20 cm and LVEF > 40%, and E wave > 0.7 m/s.
-
The hypovolemic profile was defined as the combination of subaortic VTI < 15 cm, LVEF > 40% and mitral E wave velocity < 0.7 m/s.
-
The intermediate profile referred to patients which VTI, LVEF and E wave velocity do not match with the two latter profiles.
1.8 Statistical analysis
Descriptive statistics are reported using median and interquartile range (25th to 75th percentile) for continuous variables and frequencies and proportions for categorical variables. Agreement for continuous variables was estimated using the Bland and Altman method [28]. The Bland and Altman method consists in plotting the difference between the paired measurements (expert and non-expert assessment) against their mean value. The agreement is evaluated according to the mean difference (bias), with limits of agreement defined as mean difference ± 1.96 standard deviation (SD) and outliers defined as differences considered clinically relevant. We a priori defined outliers as values equal to 20% of the normal values of the E wave velocity (1 m.s− 1) and subaortic VTI (20 cm), i.e., 0.2 m.s− 1 and 4 cm respectively. Agreement for categorical variables (LVEF above or below 40% and pleural effusion) was illustrated with percentages of specific agreement. Inter-rater reliability between experts and non-experts was calculated for continuous variables (E wave velocity and subaortic VTI) using the intra-class correlation coefficient (ICC). ICC estimates were calculated with a single-rating, absolute-agreement, 2-way mixed-effects model [29]. Reliability ranges between 0 and 1, with values closer to 1 representing stronger reliability. Reliability was considered poor for values < 0.5, moderate between 0.5 and 0.75, good between 0.75 and 0.9, and excellent > 0.90 [29]. For categorical variables, reliability was studied with Cohen’s Kappa coefficient which ranges from − 1 (total disagreement) to 1 (perfect agreement), where 0 indicates no agreement [30] and interpreted according to the Landis and Koch convention [31]. 3 Hemodynamic profiles were defined based on echocardiographic data evaluated by experts. Although sample size for Bland and Altman type studies are given in the literature [32], as expected standard deviation is difficult to evaluate and can result in a wide range of sample sizes, we did not apply this method to determine our sample size. Sample size was calculated on an expected ICC rho of 0.85 [33] with an accuracy of 0.1 (+/- 10%), a desired alpha and power for hypothesis testing of respectively 0.05 and 0.8, therefore requiring 120 patients [34]. All statistical analyses were performed using R software [35] (version 3.3.2).
2 Results
2.1 Patient characteristics
From March 30, 2018, to March 1, 2020, 55 patients were screened and 53 patients were included (one non-echogenic patient and one obese patient in whom the echography did not reveal ascites). The anticipated sample size was not reached for several reasons: the departure of the principal investigator from the hospital and because of the SARS-CoV-2 pandemic. During this period, the gastroenterology ward ultrasound device was requisitioned for the ICU and intensivists were less available to perform the expert TTE exam. Study population characteristics at admission and the characteristics of liver disease are shown in Table 1; the management of decompensated cirrhosis and status at day 28 are shown in Table 2.
2.2 Agreement and reliability
Full data (both raters) were available in 50 patients for subaortic VTI and 53 patients for E wave velocity. For subaortic VTI, the mean difference between intensivists’ and gastroenterologists’ assessment was 0.33 cm (bias 95% CI [-0.67; 1.33]) with limits of agreement between − 6.6 and 7.2 cm, while for E wave velocity, the mean difference was − 0.11 m.s− 1 (bias 95% CI [-0.18; -0.04]) with an upper and a lower limit of agreement of -0.6 and 0.37 m.s− 1 respectively. The Bland and Altman plots are shown in Fig. 1A and B. For subaortic VTI, 11/50 inter-rater measurements (22%, 95% CI [12; 36]) were considered as outliers (difference between expert and non-expert > 4 cm) and 13/53 (25%, 95% CI [14; 38]) were considered as outliers for E wave velocity (difference > 0.2 m.s− 1). Reliability for subaortic VTI was good (ICC = 0.79, 95% CI [0.58; 0.90] and moderate for E wave velocity (ICC = 0.53, 95% CI [0.19; 0.74]). As experts recorded a LVEF > 40% for all patients, agreement and reliability could not be assessed for LVEF. Pleural effusion was diagnosed in 22 patients by intensivists and in 21 patients by gastroenterologists with agreement for 42 out of 53 patients (specific agreement 79%; 95% CI [67; 88]). The Cohen’s Kappa coefficient was 0.34 (95% CI [0.57; 0.79]), indicating fair reliability.
2.3 Hemodynamic profiles
Echocardiographic characteristics evaluated by experts are shown in Table 3. As systolic function was normal in the total cohort (visual LVEF > 40% and TDI S wave velocity = 13 cm.s-1 [12; 15]), we used subaortic VTI and E wave velocity to define three hemodynamic profiles as previously described. The reliability was acceptable for subaortic VTI but not for E wave velocity.
Table 4 summarizes the hemodynamic, biological and clinical features for the three groups and associated outcomes and Fig. 2 illustrates outcome at day 28.
3 Discussion
3.1 Agreement and reliability for hemodynamics parameters
In previous studies evaluating hemodynamics and cardiac function of cirrhotic patients, echocardiography has been performed by an expert physician (cardiologist or intensivist) and never by gastroenterologists [36,37,38]. Our results suggest that echocardiography can be performed by trained gastroenterologists. However, the number of outliers is quite high (22% for subaortic VTI and 24.5% for E wave velocity) with wide limits of agreement. This is consistent with other studies where analogous or even wider limits are reported [33, 39]. It is not clear in these studies how many patients were affected by clinically significant differences. Performance of emergency physicians and intensivists has been shown to improve with more experience and training [21, 40, 41].
As reliability is good for subaortic VTI, TTE represents an informative tool despite the relatively high percentage of outliers. Discrepancies between agreement and reliability are well described in the literature, especially when there is high variability of the measured object, which has little influence on reliability but can strongly influence agreement [30]. The E wave velocity seems to be a more challenging parameter, as concordance between intensivists and gastroenterologists was poor. Thus, E wave velocity measurement may require greater expertise than subaortic VTI. A possible explanation is the risk, with a low-trained operator, of confusion between E and A waves. Moreover, the recommended theoretical training is 100 TTE in 6 months, of which 25 TTE are supervised by an expert. Such training couldn’t be put into practice here due to lack of time. These data suggest that this training may not be shortened to ensure that TTE can be reliably used by gastroenterologists.
3.2 Pleural effusion and ascites assessment by POCUS
Quantification of ascites and pleural effusion is important for evaluating fluid retention. and for the diagnosis of hepatopulmonary syndrome by performing a bubble test, especially in patients waiting for transplantation [42]. This is also true in gastroenterology ward for ascites paracentesis [43]. In ICU patients in one large observational trial, POCUS (in particular coupling echocardiography and lung ultrasound) influenced patient management in 85% of cases [44]. It has also proven useful to improve diagnostic performance where clinical evaluation has failed [45]. Therefore, POCUS (coupling TTE with pleural and abdominal ultrasound) represents an interesting tool to optimize the hemodynamic state in patients with decompensated cirrhosis with ascites. In our cohort, specific agreement was good (79%) for pleural effusion evaluation, while reliability was only fair. With longer training, gastroenterologists are likely to be able to accurately diagnose pleural effusion via lung ultrasound.
3.3 Hemodynamic profiles
Hemodynamic alterations during cirrhosis are characterized by increased total blood volume and splanchnic blood volume, decreased central blood volume (effective venous return), low systemic vascular resistance, heart rate increase, and increased cardiac output and arterial compliance [5]. This results in a hyperdynamic state and relative hypovolemia [46], emphasizing the importance of blood volume assessment during cirrhosis decompensation. Stroke volume assessment by TTE is key for blood volume evaluation [6]. The role of TTE in the management of decompensated cirrhosis with acute kidney injury has been studied in one case report [36]. However, hemodynamic profiles of cirrhotic patients derived from TTE have not been described. Our results identify distinct echocardiographic profiles in this population. Subaortic VTI seems to be the easiest parameter to asses due to the good reliability. Moreover, the association of preserved LVEF (visual assessment) and low subaortic VTI value has a good accuracy to predict hypovolemia [27]. Hemodynamic assessment is therefore possible by gastroenterologists based on subaortic VTI and LVEF with a lower level of competence than intensivists. The evaluation of the E wave requires more training to be integrated into the reasoning.
It could be objected that this simplified hemodynamic assessment could appear non-adapted to complex situations and cannot summarize all hemodynamic conditions observed in critically ill patients, especially during cirrhosis. This was a deliberate choice because we hypothesized it could facilitate assessment by non-ICU physicians. Moreover, this simple method is used in ICU by trained physicians, especially for hypovolemic or hyperkinetic profiles as demonstrated by Geri et al. [27].
In our cohort, hypovolemia was the least common profile (24% of cases) but seemed to be associated with a worse prognosis (Fig. 2). Patients in the Hyperkinetic group had the highest subaortic VTI and E wave velocities, suggesting high blood volume associated to systemic vasodilation. In this group, the median E wave velocity was 0.85 compared to 0.77 in the Intermediate group and 0.46 m.s− 1 in the Hypovolemic group. Likewise, hospital discharge at day 28 was numerically higher in the Hyperkinetic group, but again, the sub-population is too small to draw firm conclusions. Interestingly, therapeutics were similar in the three groups, especially fluid and diuretics, which were given regardless of hemodynamic profile. This reveals a systematic management rather than personalized therapeutic management according to clinical assessment. We hypothesize that diuretics are more appropriate for hyperkinetic patients and detrimental in hypovolemic group. This could explain the high mortality observed in hypovolemic group. Again, the very low number of patients in this subgroup does not allow a definitive conclusion.
Interestingly, MELD score and Child-Pugh scores were less effective to discriminate patient outcome, especially between the Intermediate and the Hyperkinetic groups. Further studies are required to evaluate the clinical benefit of a strategy based on ultrasound assessment to guide therapy, as it is done in ICU.
3.4 Limitations
Our study has several limitations. First, the planned sample size was not reached leading to an underpowered study. Second, this cohort did not include patients with LVEF below 40%. Indeed, the cardiac impairment in cirrhosis is mostly responsible for an impairment of diastolic function, which is not assessed by LVEF. Finally, longer training courses may be necessary to reliably assess echocardiographic parameters such as E wave velocity.
4 Conclusion
This study shows that a short training period allows good reliability for subaortic VTI evaluation by gastroenterologist. However, agreement is insufficient, with over 20% of clinically significant differences between intensivists and gastroenterologists for subaortic VTI and E wave velocity assessment. Nevertheless, TTE evaluation by intensivists defined three different hemodynamic profiles: Hypovolemic, Hyperkinetic, and Intermediate, with distinct prognosis and for whom different therapeutic managements could be expected. Hemodynamic assessment is therefore possible by gastroenterologists based on subaortic VTI and LVEF with a short training course. However, a full assessment including the evaluation of the E wave requires prolonged training to be integrated into the reasoning. Further studies are needed to confirm these findings and evaluate the clinical benefit of an ultrasound-based management of decompensated cirrhosis.
Data Availability
The data supporting this study are available from the corresponding author upon reasonable request.
Abbreviations
- ALAT:
-
Alanine Aminotransferase
- ALP:
-
Alkaline Phosphatase
- ASAT:
-
Aspartate Aminotransferase
- GGT:
-
Gamma Glutamyl Transferase
- ICC:
-
Intraclass Correlation Coefficient
- ICU:
-
Intensive Care Unit
- INR:
-
International Normalized Ratio
- LVEF:
-
Left Ventricular Ejection Fraction
- MELD:
-
Model for End stage Liver Disease
- POCUS:
-
Point of Care Ultrasound
- PR:
-
Prothrombin ratio
- SD:
-
Standard Deviation
- TAPSE:
-
Tricuspid Annular Plane Systolic Excursion
- TAPSV:
-
Tricuspid Annular Plane Systolic Velocity
- POCUS:
-
Point Of Care Ultrasound
- TDI:
-
Tissue Doppler Imaging
- TTE:
-
Transthoracic Echocardiography
- US:
-
Ultrasound
- VTI:
-
Velocity Time Integral
References
D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol janv. 2006;44(1):217–31.
Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatol Baltim Md févr. 1987;7(1):122–8.
Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis févr. 2008;28(1):26–42.
Turco L, Garcia-Tsao G, Magnani I, Bianchini M, Costetti M, Caporali C, et al. Cardiopulmonary hemodynamics and C-reactive protein as prognostic indicators in compensated and decompensated cirrhosis. J Hepatol mai. 2018;68(5):949–58.
Møller S, Henriksen JH, Bendtsen F. Extrahepatic complications to cirrhosis and portal hypertension: haemodynamic and homeostatic aspects. World J Gastroenterol 14 nov. 2014;20(42):15499–517.
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med déc. 2014;40(12):1795–815.
Robba C, Wong A, Poole D, Al Tayar A, Arntfield RT, Chew MS, et al. Basic ultrasound head-to-toe skills for intensivists in the general and neuro intensive care unit population: consensus and expert recommendations of the European Society of Intensive Care Medicine. Intensive Care Med déc. 2021;47(12):1347–67.
Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med 24 févr. 2011;364(8):749–57.
Perez-Avraham G, Kobal SL, Etzion O, Novack V, Wolak T, Liel-Cohen N, et al. Left ventricular geometric abnormality screening in hypertensive patients using a hand-carried ultrasound device. J Clin Hypertens Greenwich Conn mars. 2010;12(3):181–6.
Mjølstad OC, Andersen GN, Dalen H, Graven T, Skjetne K, Kleinau JO, et al. Feasibility and reliability of point-of-care pocket-size echocardiography performed by medical residents. Eur Heart J Cardiovasc Imaging déc. 2013;14(12):1195–202.
Vignon P, Dugard A, Abraham J, Belcour D, Gondran G, Pepino F, et al. Focused training for goal-oriented hand-held echocardiography performed by noncardiologist residents in the intensive care unit. Intensive Care Med oct. 2007;33(10):1795–9.
Nguyen VTQ, Ho JE, Ho CY, Givertz MM, Stevenson LW. Handheld echocardiography offers rapid assessment of clinical volume status. Am Heart J sept. 2008;156(3):537–42.
Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85.
Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatol Baltim Md févr. 2001;33(2):464–70.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 1 avr. 2009;42(2):377–81.
Mayo PH, Beaulieu Y, Doelken P, Feller-Kopman D, Harrod C, Kaplan A, et al. American college of chest Physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest avr. 2009;135(4):1050–60.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr janv. 2015;28(1):1–39e14.
Lashin H, Shepherd S, Smith A. Contrast-enhanced Echocardiography Application in Patients supported by extracorporeal membrane oxygenation (ECMO): a narrative review. J Cardiothorac Vasc Anesth juill. 2022;36(7):2080–9.
Gudmundsson P, Rydberg E, Winter R, Willenheimer R. Visually estimated left ventricular ejection fraction by echocardiography is closely correlated with formal quantitative methods. Int J Cardiol 25 mai. 2005;101(2):209–12.
Vieillard-Baron A, Charron C, Chergui K, Peyrouset O, Jardin F. Bedside echocardiographic evaluation of hemodynamics in sepsis: is a qualitative evaluation sufficient? Intensive Care Med oct. 2006;32(10):1547–52.
Bergenzaun L, Gudmundsson P, Öhlin H, Düring J, Ersson A, Ihrman L, et al. Assessing left ventricular systolic function in shock: evaluation of echocardiographic parameters in intensive care. Crit Care Lond Engl 16 août. 2011;15(4):R200.
Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, et al. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care Lond Engl 8 oct. 2012;16(5):R188.
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med avr. 2012;38(4):577–91.
Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology janv. 2004;100(1):9–15.
Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hörmann MF, Grabenwöger F. Quantification of pleural effusions: sonography versus radiography. Radiol juin. 1994;191(3):681–4.
Frankel HL, Kirkpatrick AW, Elbarbary M, Blaivas M, Desai H, Evans D, et al. Guidelines for the appropriate use of Bedside General and Cardiac Ultrasonography in the evaluation of critically Ill Patients-Part I: General Ultrasonography. Crit Care Med nov. 2015;43(11):2479–502.
Geri G, Vignon P, Aubry A, Fedou AL, Charron C, Silva S, et al. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: a post hoc analysis. Intensive Care Med mai. 2019;45(5):657–67.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet Lond Engl 8 févr. 1986;1(8476):307–10.
Koo TK, Li MY. A Guideline of selecting and reporting Intraclass correlation coefficients for Reliability Research. J Chiropr Med juin. 2016;15(2):155–63.
Cohen J. A coefficient of Agreement for Nominal Scales. Educ Psychol Meas. 1960;20(1):37–46.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics mars. 1977;33(1):159–74.
Lu MJ, Zhong WH, Liu YX, Miao HZ, Li YC, Ji MH. Sample Size for Assessing Agreement between Two Methods of Measurement by Bland-Altman Method.Int J Biostat. 1 nov2016;12(2):/j/ijb.2016.12.issue-2/ijb-2015-0039/ijb-2015-0039.xml.
Koster G, Kaufmann T, Hiemstra B, Wiersema R, Vos ME, Dijkhuizen D, et al. Feasibility of cardiac output measurements in critically ill patients by medical students. Ultrasound J 8 janv. 2020;12(1):1.
Zou GY. Sample size formulas for estimating intraclass correlation coefficients with precision and assurance. Stat Med 20 déc. 2012;31(29):3972–81.
R Core Team. A language and environment for statistical computing.R Found Stat Comput Vienna Austria [Internet]. 2016; Disponible sur: https://www.R-project.org/
Huggins JT, Doelken P, Walters C, Rockey DC. Point-of-Care Echocardiography improves Assessment of volume status in cirrhosis and Hepatorenal Syndrome. Am J Med Sci mai. 2016;351(5):550–3.
Karagiannakis DS, Vlachogiannakos J, Anastasiadis G, Vafiadis-Zouboulis I, Ladas SD. Diastolic cardiac dysfunction is a predictor of dismal prognosis in patients with liver cirrhosis. Hepatol Int oct. 2014;8(4):588–94.
Dadhich S, Goswami A, Jain VK, Gahlot A, Kulamarva G, Bhargava N. Cardiac dysfunction in cirrhotic portal hypertension with or without ascites. Ann Gastroenterol. 2014;27(3):244–9.
Dinh VA, Ko HS, Rao R, Bansal RC, Smith DD, Kim TE, et al. Measuring cardiac index with a focused cardiac ultrasound examination in the ED. Am J Emerg Med nov. 2012;30(9):1845–51.
Charron C, Prat G, Caille V, Belliard G, Lefèvre M, Aegerter P, et al. Validation of a skills assessment scoring system for transesophageal echocardiographic monitoring of hemodynamics. Intensive Care Med oct. 2007;33(10):1712–8.
Jozwiak M, Mercado P, Teboul JL, Benmalek A, Gimenez J, Dépret F et al. What is the lowest change in cardiac output that transthoracic echocardiography can detect? Crit Care Lond Engl. 11 avr 2019;23(1):116.
Levitov A, Frankel HL, Blaivas M, Kirkpatrick AW, Su E, Evans D, et al. Guidelines for the appropriate use of Bedside General and Cardiac Ultrasonography in the evaluation of critically Ill Patients-Part II: Cardiac Ultrasonography. Crit Care Med juin. 2016;44(6):1206–27.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the study of the liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol août. 2018;69(2):406–60.
Zieleskiewicz L, Muller L, Lakhal K, Meresse Z, Arbelot C, Bertrand PM, et al. Point-of-care ultrasound in intensive care units: assessment of 1073 procedures in a multicentric, prospective, observational study. Intensive Care Med sept. 2015;41(9):1638–47.
Bossone E, DiGiovine B, Watts S, Marcovitz PA, Carey L, Watts C, et al. Range and prevalence of cardiac abnormalities in patients hospitalized in a medical ICU. Chest oct. 2002;122(4):1370–6.
Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatol Baltim Md oct. 1988;8(5):1151–7.
Acknowledgements
We wish to thank all the doctors who participated in this work and made it possible: Bénédicte Brunau-Gagniard1, Valérie Phoutthasang1, Camille Bories1, Aurel Buzancais2, Martin Mahul2, Caroline Boutin2, Stéphanie Bulyez2, Guillaume Louart2, Pierre Barbaste2.
We are also grateful to our clinical research associate Lyamin Bendjeddou for his help in collecting data and Sarah Kabani for substantive editing assistance.
Author information
Authors and Affiliations
Contributions
Authors’ contribution: study concept and design : AP, JFB, CR, LM ; acquisition of data : AP, JFB, BL, LC, AD, JYL, LM, CR ; analysis and interpretation of data : BL, CR, LM; drafting of the manuscript : AP, LM, BL, CR ; critical revision of the manuscript for important intellectual content : JFB, CR, LM, CR, PP ; statistical analysis : BL ; obtained funding; administrative, technical, or material support; study supervision : JFB, PP.
Corresponding author
Ethics declarations
Conflict of Interests
None relating to this manuscript.
Financial support
Financial support from the academic institution, none from the industry.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prost, A., Bourgaux, J.F., Louart, B. et al. Echocardiographic hemodynamic assessment in decompensated cirrhosis: comparison between Intensivists and Gastroenterologists. J Clin Monit Comput 37, 1219–1228 (2023). https://doi.org/10.1007/s10877-023-00983-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-023-00983-w