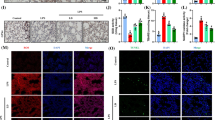
Abstract
Klebsiella pneumoniae is a Gram-negative bacterium and the causative agent of several life-threatening nosocomial infections, including pneumonia. K. pneumoniae induces acute lung injury and inflammation in humans that require immediate hospitalization and treatment. Therefore, attenuation of K. pneumoniae-induced inflammation is necessary for the survival of patients. This study investigated the mechanisms by which melatonin abrogated K. pneumoniae-induced inflammation and apoptosis of lung cell lines, HLF-1 and BEAS-2B. Our results showed that in vitro infection of HLF-1 and BEAS-2B cells by K. pneumoniae significantly induced inflammation and apoptosis increased elevated levels of IL-6, CXCL1, CXCL2, and caspase-9 mRNA. However, these effects were abrogated by melatonin treatment. Infection with K. pneumoniae significantly increased the expression of AMP-induced protein kinase (AMPK). Furthermore, AMPK silencing significantly abrogated the suppression of inflammation and apoptosis in melatonin-infected K. pneumoniae lung cells. Melatonin could alleviate K. pneumoniae infection-induced inflammation in three-dimensional lung spheroids. In conclusion, our study demonstrated that melatonin abrogated K. pneumoniae-induced inflammation and apoptosis in lung cells through AMPK. Our study demonstrated the potential of melatonin for therapy against K. pneumoniae infections including pneumonia.
Similar content being viewed by others
Introduction
Klebsiella pneumoniae (K. pneumoniae) is a Gram-negative bacterium that causes life-threatening nosocomial infections, including pneumonia (Wyres et al. 2020). In humans, K. pneumoniae is mainly located in the gastrointestinal tract and some locate in the nasopharynx, through which bacteria can enter the bloodstream or other tissues and cause infection (Wyres et al. 2020). To date, four virulent factors have been identified, including pili, capsule, lipopolysaccharide (LPS), and iron carriers (Martin et al. 2018). Importantly, K. pneumoniae is assembled by adhesins, type 1 and type 3 pili, and it promotes bacterial adhesion to epithelial, immune cells, and abiotic surfaces (Martin et al. 2018). K. pneumoniae induces acute lung injury in humans and is associated with high mortality rates of nearly 50% (Wyres et al. 2020). It is difficult to treat pneumonia because K. pneumoniae is highly resistant to carbapenems, the last resort antibiotic against Gram-negative bacteria (Chong et al. 2018). K. pneumoniae acquires antimicrobial resistance (AMR) genes that cause multidrug resistance through de novo mutations or the acquisition of plasmids and transferable genetic elements (Vardakas et al. 2018). Therefore, treatment of K. pneumoniae infections require the development of drugs that bypass AMR genes.
Pathogenic bacteria induce acute or chronic inflammation of various human organs, including the intestine and lungs (Cao 2017). For example, invasive pathogens induce inflammation by disrupting the intestinal niche and gut equilibrium generated by the commensal bacterial communities (Abt and Pamer, 2014). The in vitro intestinal organoid model demonstrated that Salmonella infections-induced intestinal cell death via inflammation (Yin and Zhou 2018). Chronic inflammation by Helicobacter pylori causes peptic ulcer disease and gastric cancer (Lehours and Ferrero 2019). Inflammation could also induce serious cell injury through different mechanisms, including the mTOR/STAT3 pathway activation, autophagy, and induction of oxidative stress (Ryter 2021). Therefore, attenuation of inflammation is critical to alleviating disease caused by bacterial infections.
Melatonin (N-acetyl-5-methoxy-tryptamine) is a sleep hormone that was originally discovered in plants (Bhattacharya et al. 2019). In humans, melatonin influences several biological activities such as circadian rhythms, mood, sleep, body temperature, locomotor activity, food intake, retina physiology, sexual behavior, seasonal reproduction, and the immune system (Bhattacharya et al. 2019). Increasing evidence has shown that melatonin exerts important regulatory effects on pathogens, including viruses and bacteria (Bahrampour Juybari et al. 2020; Anderson and Reiter 2020). For example, melatonin also exhibits broad antiviral activity against COVID-19 (Bahrampour Juybari et al. 2020), the influenza virus (Anderson and Reiter 2020), and the Ebola virus (Anderson et al. 2015). Several studies have also shown the antibacterial properties of melatonin. For example, melatonin overcomes mobilized colistin resistance (MCR) of Gram-negative pathogens (Liu et al. 2020). Melatonin also demonstrates anti-inflammatory and cytoprotective functions. For example, in the mouse model, melatonin alleviated adipose inflammation by increasing alpha-ketoglutarate levels and diverting adipose-derived exosomes to macrophages (Liu et al. 2018). Melatonin has also been reported to regulate T-cell differentiation, interfere with T/B cell interaction, and attenuate the production of pro-inflammatory factors, achieve its antioxidant action via specific receptors to exert immunomodulatory effects (Yildirim et al. 2019). In bacterial meningitis (BM), melatonin was also found to exert several protective effects in BM through various mechanisms, including the immune response, antibacterial activity, protection of the integrity of the blood–brain barrier (BBB), free radical scavenging, anti-inflammatory activity, activation of signaling pathways, and effects on the gut microbiome (Gao et al. 2021). However, the effects of melatonin on inflammation and cell death induced by bacterial infections have not been reported. Therefore, it is attractive to investigate the effects of melatonin on bacteria-induced inflammation.
In the present study, we investigated the effects of melatonin on lung epithelial cell inflammation and cytotoxicity induced by infection with K. pneumoniae and the underlying mechanisms. Importantly, an advanced in vitro three-dimensional (3D) lung spheroid model was used in the study, since it has a substantial advantage compared to conventional two-dimensional (2D) cell line models, including improved mimicking of a 3D structure in vitro. We believe that our study provides new information for understanding the pathogenesis of K. pneumoniae infections.
Materials and methods
Preparation of bacteria
K. pneumoniae was obtained from the Biological Resource center of Tianjin First Central Hospital and grown as described previously (Anand et al. 2020). K. pneumoniae was cultured in the microbial culture room and grown in Luria Bertani (LB) broth with constant shaking at 37 °C or solid media containing LB and 1.5% agar in an incubator maintained at 5% CO2, pH 7.0–7.4, and 37 °C. The bacteria grown overnight in the exponential phase were harvested by centrifugation at 2700× g for 20 min and resuspended in PBS.
Culturing of lung cell lines
HLF-1 (human lung fibroblast) cells and BEAS-2B cells (normal human bronchial epithelial cells) were purchased from Zishi Biotechnology (Shanghai, China) and cultured in Dulbecco’s modified eagle medium (DMEM, Cat No. 11054001, Thermo Fisher, Carthage, USA) containing 10% fetal bovine serum (FBS, Cat No. 12664025C, Thermo Fisher), 1% penicillin–streptomycin (Cat No. 15140163, Thermo Fisher, Carthage, USA), and 1% 2 mM L-glutamine (Cat No. 25030081, Thermo Fisher, Carthage, USA) at 37 °C and 5% CO2.
Culturing of three-dimensional (3D) lung spheroids
3D lung spheroids were purchased from Innovation Biotechnology (Tianjin, China). Culture of 3D lung spheroids was used previously published method (Kumari et al. 2020). Briefly, a mixture of 3000 HLF-1 cells and 3000 BEAS-2B cells were plated on 96-well ultra-low attachment plates (Corning) in complete media (DMEM media containing 10% FBS, 1% penicillin–streptomycin, and 1% 2 mM L-glutamine) supplemented with 4% Matrigel™ (Corning, New York, USA). Media was refreshed every 4 days by carefully aspirating 50% of the well volume and replacing with fresh complete media containing 1% Matrigel™. The 3D spheroids were re-fed with fresh media every 2 or 3 days.
Bacterial infection
HLF-1 or BEAS-2B cells or 3D lung spheroids grown in DMEM medium at 5% CO2 and 37 °C were serum-starved for 18 h and infected with the bacterial inoculum prepared in DMEM. Briefly, 80–90% confluent HLF-1 or BEAS-2B cells were seeded in 24-well plates at a density of 2 × 105 cells per well or 3D lung spheroids were seeded in a 4% Matrigel coated 24-well plate at a density of 50 spheroids per well and infected with K. pneumoniae at a multiplicity of infection (MOI) ranging from 5:1 to 10:1. The cells or 3D lung spheroids were then centrifuged for 4 min at 200× g at 22 °C and incubated between 2 and 24 h at 37 °C and 5% CO2 in a humidified incubator. HLF-1 or BEAS-2B cells infected with K. pneumoniae or 3D lung spheroids were treated with 1 μM melatonin for 24 h.
CCK-8 assay of cell viability
Lung cell viability was analyzed using the CCK8 assay kit (Beyotime, Cat. No. C0037, Shanghai, China) according to the manufacturer’s instructions. Briefly, 1 × 104 HLF-1 or BEAS-2B cells were seeded in 96-well culture plates, infected with K. pneumoniae, and treated with or without 1 μM melatonin for specified periods of time. The cells were then incubated for 4 h with 100 μL CCK-8 and the absorbance was measured at 450 nm. Cell viability (%) = OD (treated cells)/OD (control cells) × 100.
Western blotting
Protein lysates were prepared from HLF-1 and BEAS-2B cells using RIPA (catalog#P0013B, Beyotime Biotechnology, Shanghai, China). Equal amounts of protein lysates (30 µg/lane) were separated on a 12% polyacrylamide gel, transferred to PVDF membranes, and blocked with 5% FBS at room temperature for 1 h. The blots were then incubated overnight at 4 °C with the primary rabbit AMPK alpha 1 polyclonal antibody (1:1000, Cat. No. ab3759, Abcam, Cambridge, UK), anti-IL-6 antibody (1:1000, Cat. No. Ab233706, Cambridge, UK), anti-CXCL1 antibody (1:1000, Cat. No. ab124731, Cambridge, UK), anti-CXCL2 antibody (1:1000, Cat. No. ab275879, Cambridge, UK), and anti-GAPDH antibody (1:1000, Cat. No. ab8245, Abcam, Cambridge, UK). The membranes were washed with TBST for 10 min and incubated with the anti-rabbit secondary antibody (1:1000, Cat. No. ab288151, Abcam, Cambridge, UK) for 30 min at room temperature. Finally, membranes were developed using the enhanced chemiluminescence (ECL) agent (Cat. No. P0018M, Beyotime Biotechnology, Shanghai, US). The protein bands were then developed and visualized using ChemiDoc XRS + System (BIO-RAD, Cat. No. 1708265, California, US).
Reverse transcription-polymerase chain reaction (RT-qPCR)
Total RNA was extracted from lung cells using the RNeasy plus Mini Kit (Qiagen, Maryland, USA). Reverse transcription (RT) was performed with 500 ng of total RNA using the BeyoRT™ III cDNA synthesis kit (Cat. No. D7178M, Beyotime, Shanghai, China). qPCR was performed with the cDNA samples using the TB Green qPCR Kit (Takara) in the 7500 Fast Real-Time PCR System (Applied Biosystem, CA, USA). The qPCR reaction conditions included initial denaturation at 94 °C for 5 min followed by 40 cycles of amplification at 94 °C for 30 s, 58 °C for 30 s and 72 °C for 30 s. The reaction was stopped at 25 °C for 5 min. Relative gene expression levels were analyzed using the 2−ΔΔCt method. GAPDH was used as an endogenous control. RT-PCR primers used in this study are listed in Table 1.
Flow cytometry
For analysis of apoptosis rates, after treatments, 3D lung spheroids were harvested to 15 centrifuge tubes, followed by spinning down at 100 g for 5 min. Then, supernatant was discarded, followed by adding 5 mL TrypLE™ Express Enzyme (1×, phenol red, Thermo Fisher Scientific GmbH, Waltham, Massachusetts, US) and incubating in water bath (°C) for 10 min. Then, dissociation was terminated by adding 5 mL FBS. Then, single cells were washed with PBS for three times, followed by being resuspended in 1× annexin binding buffer (10× , 0.1M hepes (pH 7.4), 1.4M NaCl, 25 mM CaCl2) and incubated for 5 min at room temperature. Afterwards, cells were pelleted and stained with annexin-V-FITC (Miltenyi Biotech, Bergisch Gladbach, Germany) and propidium iodide (PI) solution (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer’s manual. They were incubated for 15 min at room temperature before an additional 300 μl Annexin V Binding Buffer was added to each sample. Gating parameters were determined using Veh/PBS-treated cells as a negative control. Unstained cells were run to account for auto fluorescence. Stained cells were analyzed by flow cytometry using a FACSLyric system (BD Biosciences, Franklin Lakes, New Jersey, US). For instrument setup, the excitation wavelength was set to 488 nm, and emission wavelength was set to 530 for annexin-V, and the excitation wavelength was set to 488 nm, and emission wavelength was set to 585 for PI.
Statistical analysis
GraphPad Prism (version 9, GraphPad Software, San Diego, CA, US) was used for statistical analysis. Data are shown as mean ± SEM. One-way analysis of variance (ANOVA) was used to compare the data between multiple groups. P < 0.05 was considered statistically significant.
Results
K. pneumoniae-induced inflammation in lung cell lines
We analyzed the inflammation induced by K. pneumoniae in the lung cell lines. Infection of the HLF-1 and BEAS-2B lung cell lines with K. pneumoniae (MOI = 10) significantly increased the mRNA levels of inflammation-related genes such as IL-6 (Fig. 1A, D), CXCL1 (Fig. 1B, E), and CXCL2 (Fig. 1C, F). This demonstrated that K. pneumoniae-induced inflammation in lung cells.
K. pneumoniae infection induces inflammation in lung cell lines. K. pneumoniae infection significantly increased the mRNA expression of A IL-6, B CXCL1, and C CXCL2 in the HLF-1 cell line. K. pneumoniae infection significantly increased the protein levels of D IL-6, E CXCL1, and F CXCL2 in the HLF-1 cell line. Infection with K. pneumoniae significantly increased the mRNA expression of G IL-6, H CXCL1, and I CXCL2 in the BEAS-2B cell line. K. pneumoniae infection significantly increased the protein levels of J IL-6, K CXCL1, and L CXCL2 in the BEAS-2B cell line
Infection with K. pneumoniae significantly reduced the viability of lung cells
Lung injury and inflammation are the common characteristics of lung diseases (Daniel et al. 2019). Therefore, we investigated the effects of K. pneumoniae infection on lung cell viability. K. pneumoniae infection (MOI = 5:1 and 10:1) significantly decreased the viability of HLF-1 and BEAS-2B cells (Fig. 2A, C). Furthermore, HLF-1 and BEAS-2B cells infected with K. pneumoniae (MOI = 5:1 and 10:1, respectively) showed significantly higher levels of caspase-9 mRNA, a pro-apoptotic gene (Fig. 2B, D). This demonstrated that infection with K. pneumoniae-induced apoptosis in lung cells.
Infection with K. pneumoniae significantly reduced the viability of lung cell lines. K. pneumoniae infection (MOI = 5:1 and 10:1) significantly decreased the viability of A HLF-1 and C BEAS-2B cells. K. pneumoniae infection (MOI = 5:1 and 10:1) significantly increased the expression of the cellular apoptosis marker, caspase 9, in B HLF-1 and D BEAS-2B cells
Melatonin-alleviated inflammation induced by K. pneumoniae in lung cells
Melatonin reduced inflammation in several cell types (Zhang et al. 2020). IL-6, CXCL1, and CXCL2 mRNA expression in HLF-1 and BEAS-2B cells infected with K. pneumoniae treated with 1 μM melatonin was significantly lower compared to the corresponding controls (Fig. 3A–F). The dose and treatment time were referenced in previous studies (Lee et al. 2018; Rahman et al. 2005). This demonstrated that melatonin treatment significantly reduced the inflammatory response induced by K. pneumoniae infection in lung cell lines.
Melatonin alleviates inflammation induced by infection with K. pneumoniae in lung cell lines. Melatonin treatment suppressed K. pneumoniae infection-induced upregulation of A IL6, B CXCL1 and C CXCL2 mRNA expression in HLF-1 cells. Melatonin treatment suppressed K. pneumoniae infection-induced upregulation of D IL6, E CXCL1, and F CXCL2 mRNA expression in BEAS-2B cells
Melatonin treatment increased the viability of K. pneumoniae-infected lung cells
Next, we investigated the effects of melatonin on lung cell apoptosis induced by K. pneumoniae infection. Treatment of K. pneumoniae-infected lung cell lines with 1 μM melatonin significantly reduced caspase 9 expression levels (Fig. 4A, C). Furthermore, melatonin significantly increased the viability of HLF-1 and BEAS-2B cells infected with K. pneumoniae (Fig. 4B, D). These results demonstrated that melatonin significantly increased the viability of the K. pneumoniae-infected lung cell lines.
Melatonin increases the viability of K. pneumoniae-infected lung cells by suppressing apoptosis. Melatonin-suppressed K. pneumoniae infection-induced upregulation of caspase 9 expression in A HLF-1 and B BEAS-2B cells. Melatonin increased viability of C HLF-1 and D BEAS-2B cells infected with K. pneumoniae
AMPK expression was elevated in lung cells infected with K. pneumoniae
AMPK is a key regulator of cellular metabolism that plays a significant role in modulating bacterial infections (Jo et al. 2019). Therefore, we investigated whether infection by K. pneumoniae modulated AMPK expression. K. pneumoniae (MOI = 10:1) significantly increased AMPK mRNA expression in HLF-1 and BEAS-2B cells at different time points (2, 4, and 8 h) (Fig. 5A, C). Furthermore, K. pneumoniae infection significantly increased AMPK protein levels in HLF-1 and BEAS-2B cells (Fig. 5B, D). This showed that K. pneumoniae infection significantly increased AMPK mRNA and protein levels in lung cells.
Melatonin-suppressed K. pneumoniae-induced inflammation in lung cells via AMPK
We then investigated the role of AMPK in the anti-inflammatory effects of melatonin in K. pneumoniae-infected lung cells. We silenced AMPK in HLF-1 and BEAS-2B cells by transfection of cells with siRNA against AMPK (AMPK KD) (Fig. 6A, D). The qRT-PCR results indicated that the IL-6 and CXCL1 mRNA levels mRNA levels were significantly higher in AMPK-silenced lung cell lines treated with melatonin infected with K. pneumoniae compared to lung cell lines treated with melatonin infected with K. pneumoniae (Fig. 6B, C, E, F). This demonstrated that melatonin suppressed inflammation caused by K. pneumoniae infection in the lung cells via AMPK.
Melatonin-suppressed inflammation in K. pneumoniae-infected lung cells by AMPK. qRT-PCR analysis indicating AMPK mRNA levels in control cells and AMP siRNA-transfected A HLF-1 and D BEAS-2B cells. qRT-PCR analysis showing relative levels of IL-6 mRNA in control and AMPK-silenced B HLF-1 and E BEAS-2B cells treated with 1 μM melatonin and infected with K. pneumoniae. C, F qRT-PCR analysis showing relative CXCL1 mRNA levels in the control and AMPK-silenced C HLF-1 and F BEAS-2B cells treated with 1 μM melatonin and infected with K. pneumoniae. As shown, AMPK KD abrogated the anti-inflammatory effects of melatonin in K. pneumoniae-infected lung cells
Melatonin-suppressed apoptosis in lung cells infected with K. pneumoniae by AMPK
Next, we analyzed the effects of melatonin on the viability of K. pneumoniae-infected lung cells and the corresponding mechanism. Treatment of HLF-1 and BEAS-2B cells infected with K. pneumoniae with 1 μM melatonin significantly reduced caspase9 mRNA expression levels (Fig. 7A, C) and increased cell viability (Fig. 7B, D) compared to HLF-1 and BEAS-2B cells infected with K. pneumoniae without melatonin treatment. Interestingly, effects of melatonin on the promotion of caspase9 expression and reduction of cell viability by K. pneumoniae infection were found in both cell types (Fig. 7). This showed that melatonin alleviated apoptosis of K. pneumoniae-infected lung cells via AMPK.
Melatonin promotes the viability of K. pneumoniae-infected lung cells via AMPK. Melatonin treatment (1 μM) suppressed the upregulation of caspase9 mRNA levels, while AMPK knockdown attenuated the effects of melatonin in K. pneumoniae-infected A HLF-1 and C BEAS-2B cells. Melatonin treatment (1 μM) increased cell viability, while AMPK knockdown attenuated the effects of melatonin in cells infected with K. pneumoniae B HLF-1 and D BEAS-2B
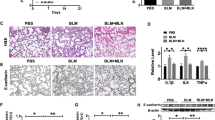
Melatonin-alleviated inflammation caused by K. pneumoniae in 3D lung spheroids
3D in vitro models have been shown to more accurately mimic in vivo physiology (Yin et al. 2021; Clevers 2016). Thus, we evaluated inflammation due to K. pneumoniae infections and the effects of melatonin on K. pneumoniae infections-induced inflammation in 3D lung spheroids (Fig. 8A). K. pneumoniae infections increased IL-6 (Fig. 8B), CXCL1 (Fig. 8C), and CXCL2 (Fig. 8D) mRNA expression in 3D lung spheroids. Importantly, 1 μM melatonin significantly reduced IL-6 (Fig. 8E), CXCL1 (Fig. 8F), and CXCL2 (Fig. 8G) mRNA expression levels in 3D lung spheroids. To further confirm the effects of K. pneumoniae infections on lung cell apoptosis and the effects of melatonin on lung cell apoptosis, the flow cytometry assays were performed to confirm cell apoptosis induced by K. pneumoniae infections, while melatonin (1 μM) attenuated K. pneumoniae infection-induced cell apoptosis in 3D lung spheroids (Fig. 8H). This demonstrated that melatonin treatment significantly reduced the inflammatory response induced by K. pneumoniae infection in a 3D lung spheroid model.
Melatonin-alleviated inflammation caused by K. pneumoniae in 3D lung spheroids. A Morphology of 3D lung spheroids. Infection with K. pneumoniae increased the expression of IL-6 B, CXCL1 C and CXCL2 D mRNA in 3D lung spheroids. Melatonin (1 μM) significantly reduced IL-6 E, CXCL1 F and CXCL2 G mRNA expression in 3D lung spheroids; flow cytometry indicating that K. pneumoniae infections-induced cell apoptosis, while melatonin (1 μM) could attenuate K. pneumoniae infection-induced cell apoptosis in 3D lung spheroids H
Discussion
K. pneumoniae was first described by Carl Friedlander in 1882 as an encapsulated bacterium isolated from the lungs of patients who died from pneumonia. K. pneumoniae is the third leading cause of healthcare-associated infections in the United States (9.9%) behind Clostridium difficile and Staphylococcus aureus (Martin and Bachman 2018). In the present study, we demonstrated that infection with K. pneumoniae-induced inflammation and cytotoxicity in two lung cell lines, HLF-1 and BEAS-2B, by increasing the expression levels of AMPK. This study also demonstrated that melatonin alleviated inflammation and cell death in lung cell lines in response to infection by K. pneumoniae. Furthermore, this study demonstrated that AMPK mediated the effects of melatonin in alleviating inflammation and cell death in K. pneumoniae-infected lung cell lines. We also confirmed that melatonin could alleviate inflammation induced by K. pneumonia infections in 3D lung spheroids.
The immune system plays a key role in protecting the human body from disease-causing pathogens such as bacteria and viruses. In addition, infections from foreign pathogens such as bacteria activate the immune response or inflammation. Pathogenic oral bacteria and related toxins induce inflammation at distant sites in the body (Martin and Bachman 2018; Han and Wang 2013). Wong et al (2014) demonstrated prostatic inflammation and fibrosis in a mouse model of chronic bacterial infection. In patients with Crohn’s disease, abnormal ileal bacterial colonization is associated with altered innate immune responses to invasive bacteria (Barnich et al. 2013). Wu et al (2018) demonstrate that the oral pathogen Salmonella enterica induced intestinal inflammation to eliminate competitors to gain exclusive access to fructose-asparagine, an essential nutrient for their growth. In the lungs, innate immunity plays an important role in the elimination of several pathogens without adverse immunopathological effects and in the absence of adaptive immunity (Dulek et al. 2014). In the lungs, bacterial pathogens, including K. pneumoniae, can induce immune responses that result in increased expression of innate immune genes such as interleukin-17A (IL-17A), IL-17F, and IL-22 (Dulek et al. 2014). In the present study, K. pneumoniae-infected lung cells showed significantly higher mRNA and protein expression of pro-inflammatory genes such as IL6, CXCL1, and CXCL2 (Fig. 1). Hoogerwerf et al. (2012) demonstrated that K. pneumoniae infection was closely associated with the inflammatory response in the lung epithelium. Therefore, it is essential to determine the mechanisms underlying the inflammatory responses during K. pneumoniae infection of lung cells. Furthermore, K. pneumoniae infection is also associated with severe lung injury (Dulek et al. 2014). In the present study, we confirm that K. pneumoniae infection-induced cellular apoptosis in lung cell lines (Fig. 2).
In humans, melatonin is produced by the pineal glands. However, melatonin is widely produced naturally in several living organisms, from bacteria to humans (Liu et al. 2020). Melatonin exerts a variety of biological functions, including circadian rhythms, sleep, immune responses, and others (Liu et al. 2020). Several studies have shown that melatonin protects against bacterial infections. Liu et al (2020) demonstrated that melatonin substantially increased the activity of colistin against mobilized colistin-resistant (MCR)-positive Gram-negative pathogens. Melatonin is a powerful antioxidant molecule that can reduce oxidative stress and up-regulate antioxidative defense mechanisms that can influence innate immunity against bacterial pathogens (Liu et al. 2020). Xu et al (2019) demonstrated that melatonin exerted its protective effects against polymicrobial sepsis in the mouse model by promoting the development of the neutrophil extracellular trap (NET), which contributed to antibacterial activity during polymicrobial infection. In the present study, we showed that melatonin suppressed inflammation and apoptosis in lung cells infected with K. pneumoniae (Figs. 3, 4, and 8).
AMPK is a highly conserved serine/threonine kinase that functions as a key metabolic sensor in eukaryotic cells and plays an important role in the adaptive response to exercise (Spaulding and Yan 2022). AMPK is associated with several biological processes, including tumorigenesis, virus infections, metabolism, and others (Prantner et al. 2017). AMPK also regulates host defenses against bacterial pathogens and is closely associated with innate immunity (Prantner et al. 2017). In this study, we demonstrated that melatonin alleviated inflammation and apoptosis related to K. pneumoniae infection in lung cells via AMPK (Figs. 6, 7, 8).
In conclusion, our study demonstrated that K. pneumoniae infection-induced inflammation and apoptosis in lung cell lines, which was associated with increased AMPK expression. Furthermore, melatonin alleviated K. pneumoniae infection-related inflammation and apoptosis of lung cell lines via AMPK. These results suggested that melatonin is a promising therapeutic agent for alleviating lung pathology and pneumonia related to K. pneumoniae infection.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abt MC, Pamer EG (2014) Commensal bacteria mediated defenses against pathogens. Curr Opin Immunol 29:16–22. https://doi.org/10.1016/j.coi.2014.03.003
Anand T, Virmani N, Kumar S, Mohanty AK, Pavulraj S, Bera BC, Vaid RK, Ahlawat U, Tripathi BN (2020) Phage therapy for treatment of virulent Klebsiella pneumoniae infection in a mouse model. J Glob Antimicrob Resist 21:34–41. https://doi.org/10.1016/j.jgar.2019.09.018
Anderson G, Maes M, Markus RP, Rodriguez M (2015) Ebola virus: melatonin as a readily available treatment option. J Med Virol 87(4):537–543. https://doi.org/10.1002/jmv.24130
Anderson G, Reiter RJ (2020) Melatonin: roles in influenza, Covid-19, and other viral infections. Rev Med Virol 30(3):e2109. https://doi.org/10.1002/rmv.2109
BahrampourJuybari K, Pourhanifeh MH, Hosseinzadeh A, Hemati K, Mehrzadi S (2020) Melatonin potentials against viral infections including COVID-19: current evidence and new findings. Virus Res 287:198108. https://doi.org/10.1016/j.virusres.2020.198108
Barnich N, Denizot J, Darfeuille-Michaud A (2013) E-coli-mediated gut inflammation in genetically predisposed Crohn’s disease patients. Pathol Biol (Paris) 61(5):65–69. https://doi.org/10.1016/j.patbio.2010.01.004
Bhattacharya S, Patel KK, Dehari D, Agrawal AK, Singh S (2019) Melatonin and its ubiquitous anticancer effects. Mol Cell Biochem 462(1–2):133–155
Cao X (2017) Intestinal inflammation induced by oral bacteria. Science 358(6361):308–309
Chong Y, Shimoda S, Shimono N (2018) Current epidemiology, genetic evolution, and clinical impact of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect Genet Evol 61:185–188
Clevers H (2016) Modeling development and disease with organoids. Cell 165(7):1586–1597. https://doi.org/10.1016/j.cell.2016.05.082
Daniel C, Leppkes M, Munoz LE, Schley G, Schett G, Herrmann M (2019) Extracellular DNA traps in inflammation, injury, and healing. Nat Rev Nephrol 15(9):559–575
Dulek DE, Newcomb DC, Goleniewska K, Cephus J, Zhou W, Reiss S, Toki S, Ye F, Zaynagetdinov R, Sherrill TP et al (2014) Allergic airway inflammation decreases lung bacterial burden following acute Klebsiella pneumoniae infection in a neutrophil- and CCL8-dependent manner. Infect Immun 82(9):3723–3739. https://doi.org/10.1128/IAI.00035-14
Gao T, Wang Z, Dong Y, Gao J, Chen Y (2021) Melatonin-mediated colonic microbiota metabolite butyrate prevents acute sleep deprivation-induced colitis in mice. Int J Mol Sci 22(21):11894. https://doi.org/10.3390/ijms222111894
Han YW, Wang X (2013) Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res 92(6):485–491. https://doi.org/10.1177/0022034513487559
Hoogerwerf JJ, van der Windt GJ, Blok DC, Hoogendijk AJ, De Vos AF, van’t VeerFlorquinKobayashi CSKS, Flavell RA, van der Poll T (2012) Interleukin-1 receptor-associated kinase M-deficient mice demonstrate an improved host defense during Gram-negative pneumonia. Mol Med 18:1067–1075
Jo EK, Silwal P, Yuk JM (2019) AMPK-targeted effector networks in mycobacterial infection. Front Microbiol 10:520. https://doi.org/10.3389/fmicb.2019.00520
Kumari N, Bhargava A, Rath SN (2020) T-type calcium channel antagonist, TTA-A2 exhibits anti-cancer properties in 3D spheroids of A549, a lung adenocarcinoma cell line. Life Sci 260:118291. https://doi.org/10.1016/j.lfs.2020.118291
Lee SJ, Lee HJ, Jung YH, Kim JS, Choi SH, Han HJ (2018) Melatonin inhibits apoptotic cell death induced by Vibrio vulnificus VvhA via melatonin receptor 2 coupling with NCF-1. Cell Death Dis 9(2):48
Lehours P, Ferrero RL (2019) Review: Helicobacter: inflammation, immunology, and vaccines. Helicobacter 24(Suppl 1):e12644. https://doi.org/10.1111/hel.12644
Liu Y, Jia Y, Yang K, Tong Z, Shi J, Li R, Xiao X, Ren W, Hardeland R, Reiter RJ et al (2020) Melatonin overcomes MCR-mediated colistin resistance in Gram-negative pathogens. Theranostics 10(23):10697–10711
Liu Z, Gan L, Zhang T, Ren Q, Sun C (2018) Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J Pineal Res. https://doi.org/10.1111/jpi.12455
Martin RM, Bachman MA (2018) Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol 22(8):4. https://doi.org/10.3389/fcimb.2018.00004
Prantner D, Perkins DJ, Vogel SN (2017) AMP-activated kinase (AMPK) promotes innate immunity and antiviral defense through modulation of stimulator of interferon genes (STING) signaling. J Biol Chem 292(1):292–304
Rahman MA, Azuma Y, Fukunaga H, Murakami T, Sugi K, Fukushi H, Miura K, Suzuki H, Shirai M (2005) Serotonin and melatonin, neurohormones for homeostasis, as novel inhibitors of infections by the intracellular parasite chlamydia. J Antimicrob Chemother 56(5):861–868. https://doi.org/10.1093/jac/dki331
Ryter SW (2021) Heme oxgenase-1, a cardinal modulator of regulated cell death and inflammation. Cells 10(3):515. https://doi.org/10.3390/cells10030515
Spaulding HR, Yan Z (2022) AMPK and the adaptation to exercise. Annu Rev Physiol 10(84):209–227. https://doi.org/10.1146/annurev-physiol-060721-095517
Vardakas KZ, Voulgaris GL, Samonis G, Falagas ME (2018) Inhaled colistin monotherapy for respiratory tract infections in adults without cystic fibrosis: a systematic review and meta-analysis. Int J Antimicrob Agents 51(1):1–9
Wong L, Hutson PR, Bushman W (2014) Prostatic inflammation induces fibrosis in a mouse model of chronic bacterial infection. PLoS ONE 9(6):e100770. https://doi.org/10.1371/journal.pone.0100770
Wu J, Sabag-Daigle A, Borton MA, Kop LFM, Szkoda BE, Deatherage Kaiser BL, Lindemann SR, Renslow RS, Wei S, Nicora CD et al (2018) Salmonella-Mediated Inflammation Eliminates Competitors for Fructose-Asparagine in the Gut. Infect Immun. https://doi.org/10.1128/IAI.00945-17
Wyres KL, Lam MMC, Holt KE (2020) Population genomics of Klebsiella pneumoniae. Nat Rev Microbiol 18(6):344–359
Xu L, Zhang W, Kwak M, Zhang L, Lee PCW, Jin JO (2019) Protective effect of melatonin against polymicrobial sepsis is mediated by the anti-bacterial effect of neutrophils. Front Immunol 10:1371. https://doi.org/10.3389/fimmu.2019.01371
Yildirim A, Arabacı Tamer S, Sahin D, Bagriacik F, Kahraman MM, Onur ND, Cayirli YB, Cilingir Kaya ÖT, Aksu B, Akdeniz E, Yuksel M, Çetinel Ş, Yeğen BÇ (2019) The effects of antibiotics and melatonin on hepato-intestinal inflammation and gut microbial dysbiosis induced by a short-term high-fat diet consumption in rats. Br J Nutr 122(8):841–855
Yin Y, Liu PY, Shi Y, Li P (2021) Single-cell sequencing and organoids: a powerful combination for modelling organ development and diseases. Rev Physiol Biochem Pharmacol 179:189–210
Yin Y, Zhou D (2018) Organoid and enteroid modeling of Salmonella infection. Front Cell Infect Microbiol 8:102. https://doi.org/10.3389/fcimb.2018.00102
Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, Liu C, Reiter RJ (2020) COVID-19: melatonin as a potential adjuvant treatment. Life Sci 250:117583. https://doi.org/10.1016/j.lfs.2020.117583
Acknowledgements
Not applicable.
Funding
This study was financially supported by the Chun Feng Scientific Research Fund Project of Tianjin First Central Hospital (Grant No. 2019CF17).
Author information
Authors and Affiliations
Contributions
WJ and WY: Study conceptualization; WJ, JL, and XZ: Data curation; WJ: Formal analysis; WY: Methodology; WY: Project administration and supervision, data validation, review, and editing of the manuscript draft; WJ: Original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
I, the undersigned, give my consent for the publication of identifiable details, which can include photographs and / or videos and/or case history and/or details within the text (“Material”) to be published in the above Journal and Article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, W., Liu, J., Zhao, X. et al. Melatonin ameliorates lung cell inflammation and apoptosis caused by Klebsiella pneumoniae via AMP-activated protein kinase. Inflammopharmacol 30, 2345–2357 (2022). https://doi.org/10.1007/s10787-022-01073-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-01073-0