Abstract
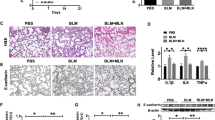
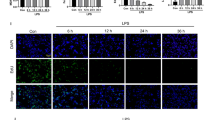
The mitochondrial quality control of lung epithelial cells is disturbed during sepsis, which contributes to abnormal mitochondrial function and acute lung injury. Melatonin is one of the primary hormones secreted by the pineal gland, displaying favorable antioxidative actions in sepsis and cardiopulmonary disease. However, the potential roles and molecular basis of melatonin in lipopolysaccharide (LPS)-treated lung epithelial cells have not been explored and reported. Herein, we investigated whether melatonin could protect against sepsis-induced acute lung injury (ALI) and LPS-treated lung epithelial cells through the mitochondrial quality control as well as its possible molecular targets. Wild type and Sirt3 knockout mice were intratracheally instilled with LPS for 12 h to construct an in vivo acute lung injury model. Both A549 lung epithelial cells and primary alveolar type II (AT-II) cells were used to explore the possible roles of melatonin in vitro by incubating with small interfering RNA against Sirt3. To determine the involvement of the melatonin receptor, cells and mice were treated with si Mtnr1b and luzindole. Melatonin pretreatment significantly inhibited pathological injury, inflammatory response, oxidative stress, and apoptosis in LPS-treated lung tissues and LPS-treated lung epithelial cells. Furthermore, melatonin also shifted the dynamic course of mitochondria from fission to fusion, inhibited mitophagy and fatty acid oxidation in LPS-treated lung epithelial cells in vitro and in vivo. However, SIRT3 inhibition abolished the protective roles of melatonin in acute lung injury. Mechanistically, we found that melatonin increased the activity and expression of SIRT3, which further promoted the deacetylation of SOD2 at K122 and K68. More importantly, melatonin exerted pulmonary protection by activating MTNR1B but not MTNR1A during ALI. Collectively, melatonin could preserve the mitochondrial quality control of lung epithelial cells through the deacetylation of SOD2 in a SIRT3-dependent manner, which eventually alleviated sepsis-induced injury, inflammation, oxidative stress, and apoptosis. Thus, melatonin may serve as a promising candidate against ALI in the future.










Similar content being viewed by others
Availability of data and materials
The data and material that support the findings of this study are available upon request to the corresponding authors.
References
Peukert K, Fox M, Schulz S, Feuerborn C, Frede S, Putensen C, Wrigge H, Kümmerer B, David S, Seeliger B, Welte T, Latz E, Klinman D, Wilhelm C, Steinhagen F, Bode C (2021) Inhibition of caspase-1 with tetracycline ameliorates acute lung injury. Am J Respir Crit Care Med 204(1):53–63
Lv H, Liu Q, Wen Z, Feng H, Deng X, Ci X (2017) Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol 12:311–324
Du M, Garcia J, Christie J, Xin J, Cai G, Meyer N, Zhu Z, Yuan Q, Zhang Z, Su L, Shen S, Dong X, Li H, Hutchinson J, Tejera P, Lin X, Wang M, Chen F, Christiani D (2021) Integrative omics provide biological and clinical insights into acute respiratory distress syndrome. Intensive Care Med 47(7):761–771
Heijnen N, Hagens L, Smit M, Cremer O, Ong D, van der Poll T, van Vught L, Scicluna B, Schnabel R, van der Horst I, Schultz M, Bergmans D, Bos L (2021) Biological Subphenotypes of Acute Respiratory Distress Syndrome Show Prognostic Enrichment in Mechanically Ventilated Patients without Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 203(12):1503–1511
Hu R, Xu H, Jiang H, Zhang Y, Sun Y (2013) The role of TLR4 in the pathogenesis of indirect acute lung injury. Front Biosci (Landmark edition) 18:1244–1255
Y. Zhou, P. Li, A. Goodwin, J. Cook, P. Halushka, E. Chang, B. Zingarelli, H. Fan, Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury, Critical care (London, England) 23(1) (2019) 44.
Bock F, Tait S (2020) Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol 21(2):85–100
Deshwal S, Fiedler K, Langer T (2020) Mitochondrial proteases: multifaceted regulators of mitochondrial plasticity. Annu Rev Biochem 89:501–528
Banoth B, Cassel S (2018) Mitochondria in innate immune signaling. Transl Res 202:52–68
Xie L, Shi F, Tan Z, Li Y, Bode A, Cao Y (2018) Mitochondrial network structure homeostasis and cell death. Cancer Sci 109(12):3686–3694
Wu Y, Yao Y, Lu Z (2019) Mitochondrial quality control mechanisms as potential therapeutic targets in sepsis-induced multiple organ failure. J Mol Med (Berl) 97(4):451–462
Zhao G, Cao K, Xu C, Sun A, Lu W, Zheng Y, Li H, Hong G, Wu B, Qiu Q, Lu Z (2017) Crosstalk between Mitochondrial Fission and Oxidative Stress in Paraquat-Induced Apoptosis in Mouse Alveolar Type II Cells. Int J Biol Sci 13(7):888–900
Hou L, Zhang J, Liu Y, Fang H, Liao L, Wang Z, Yuan J, Wang X, Sun J, Tang B, Chen H, Ye P, Ding Z, Lu H, Wang Y, Wang X (2021) MitoQ alleviates LPS-mediated acute lung injury through regulating Nrf2/Drp1 pathway. Free Radical Biol Med 165:219–228
Zhou Z, Tan E (2020) Oxidized nicotinamide adenine dinucleotide-dependent mitochondrial deacetylase sirtuin-3 as a potential therapeutic target of Parkinson’s disease. Ageing Res Rev 62:101107
Kane A, Sinclair D (2018) Sirtuins and NAD in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ Res 123(7):868–885
S. Sharma, S. Bhattarai, H. Ara, G. Sun, D. St Clair, M. Bhuiyan, C. Kevil, M. Watts, P. Dominic, T. Shimizu, K. McCarthy, H. Sun, M. Panchatcharam, S. Miriyala, SOD2 deficiency in cardiomyocytes defines defective mitochondrial bioenergetics as a cause of lethal dilated cardiomyopathy, Redox biology 37 (2020) 101740.
Pi H, Xu S, Reiter R, Guo P, Zhang L, Li Y, Li M, Cao Z, Tian L, Xie J, Zhang R, He M, Lu Y, Liu C, Duan W, Yu Z, Zhou Z (2015) SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 11(7):1037–1051
D. Kurundkar, A. Kurundkar, N. Bone, E. Becker, W. Liu, B. Chacko, V. Darley-Usmar, J. Zmijewski, V. Thannickal, SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury, JCI insight 4(1) (2019).
Lerner A, Case J, Takahashi Y (1960) Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J Biol Chem 235:1992–1997
Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, Fougerou C (2017) Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr Neuropharmacol 15(3):434–443
Boutin J, Witt-Enderby P, Sotriffer C, Zlotos D (2020) Melatonin receptor ligands: A pharmaco-chemical perspective. J Pineal Res 69(3):e12672
M. Zhai, B. Li, W. Duan, L. Jing, B. Zhang, M. Zhang, L. Yu, Z. Liu, B. Yu, K. Ren, E. Gao, Y. Yang, H. Liang, Z. Jin, S. Yu, Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis, Journal of pineal research 63(2) (2017).
Liu L, Chen H, Jin J, Tang Z, Yin P, Zhong D, Li G (2019) Melatonin ameliorates cerebral ischemia/reperfusion injury through SIRT3 activation. Life Sci 239:117036
Zhang Y, Li X, Grailer J, Wang N, Wang M, Yao J, Zhong R, Gao G, Ward P, Tan D, Li X (2016) Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J Pineal Res 60(4):405–414
Z. Ding, X. Wu, Y. Wang, S. Ji, W. Zhang, J. Kang, J. Li, G. Fei, Melatonin prevents LPS-induced epithelial-mesenchymal transition in human alveolar epithelial cells via the GSK-3β/Nrf2 pathway, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 132 (2020) 110827.
Li J, Liu L, Zhou X, Lu X, Liu X, Li G, Long J (2020) Melatonin Attenuates Sepsis-Induced Acute Lung Injury Through Improvement of Epithelial Sodium Channel-Mediated Alveolar Fluid Clearance Via Activation of SIRT1/SGK1/Nedd4-2 Signaling Pathway. Front Pharmacol 11:590652
Ning L, Wei W, Wenyang J, Rui X, Qing G (2020) Cytosolic DNA-STING-NLRP3 axis is involved in murine acute lung injury induced by lipopolysaccharide. Clin Transl Med 10(7):e228
Di S, Wang Z, Hu W, Yan X, Ma Z, Li X, Li W, Gao J (2020) The Protective Effects of Melatonin Against LPS-Induced Septic Myocardial Injury: A Potential Role of AMPK-Mediated Autophagy. Front Endocrinol (Lausanne) 11:162
Mao K, Luo P, Geng W, Xu J, Liao Y, Zhong H, Ma P, Tan Q, Xia H, Duan L, Song S, Long D, Liu Y, Yang T, Wu Y, Jin Y (2021) An Integrative Transcriptomic and Metabolomic Study Revealed That Melatonin Plays a Protective Role in Chronic Lung Inflammation by Reducing Necroptosis. Front Immunol 12:668002
Hou L, Zhang J, Liu Y, Fang H, Liao L, Wang Z, Yuan J, Wang X, Sun J, Tang B, Chen H, Ye P, Ding Z, Lu H, Wang Y, Wang X (2021) MitoQ alleviates LPS-mediated acute lung injury through regulating Nrf2/Drp1 pathway. Free Radic Biol Med 165:219–228
Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W, Tang Q (2019) STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol 24:101215
Shi J, Yu T, Song K, Du S, He S, Hu X, Li X, Li H, Dong S, Zhang Y, Xie Z, Li C, Yu J (2021) Dexmedetomidine ameliorates endotoxin-induced acute lung injury in vivo and in vitro by preserving mitochondrial dynamic equilibrium through the HIF-1a/HO-1 signaling pathway. Redox Biol 41:101954
Pérez H, Finocchietto PV, Alippe Y, Rebagliati I, Elguero ME, Villalba N, Poderoso JJ, Carreras MC (2018) p66(Shc) Inactivation Modifies RNS Production, Regulates Sirt3 Activity, and Improves Mitochondrial Homeostasis. Delaying the Aging Process in Mouse Brain, Oxid Med Cell Longev 2018:8561892
Singh C, Chhabra G, Ndiaye M, Garcia-Peterson L, Mack N, Ahmad N (2018) The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid Redox Signal 28(8):643–661
Zhu Y, Zou X, Dean A, Brien J, Gao Y, Tran E, Park S, Liu G, Kieffer M, Jiang H, Stauffer M, Hart R, Quan S, Satchell K, Horikoshi N, Bonini M, Gius D (2019) Lysine 68 acetylation directs MnSOD as a tetrameric detoxification complex versus a monomeric tumor promoter. Nat Commun 10(1):2399
Dubocovich M, Masana M, Iacob S, Sauri D (1997) Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn Schmiedebergs Arch Pharmacol 355(3):365–375
Feng Y, Huang W, Paul C, Liu X, Sadayappan S, Wang Y, Pauklin S (2021) Mitochondrial nucleoid in cardiac homeostasis: bidirectional signaling of mitochondria and nucleus in cardiac diseases. Basic Res Cardiol 116(1):49
X. Chen, R. Kang, G. Kroemer, D. Tang, Organelle-specific regulation of ferroptosis, Cell death and differentiation (2021).
J. Iovine, S. Claypool, N. Alder, Mitochondrial compartmentalization: emerging themes in structure and function, Trends in biochemical sciences (2021).
Kellner M, Noonepalle S, Lu Q, Srivastava A, Zemskov E, Black S (2017) ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv Exp Med Biol 967:105–137
Wang M, Wang K, Deng G, Liu X, Wu X, Hu H, Zhang Y, Gao W, Li Q (2020) Mitochondria-Modulating Porous Se@SiO Nanoparticles Provide Resistance to Oxidative Injury in Airway Epithelial Cells: Implications for Acute Lung Injury. Int J Nanomed 15:2287–2302
Schumacker P, Gillespie M, Nakahira K, Choi A, Crouser E, Piantadosi C, Bhattacharya J (2014) Mitochondria in lung biology and pathology: more than just a powerhouse, American journal of physiology. Lung cellular and molecular physiology 306(11):L962–L974
Palmeira C, Teodoro J, Amorim J, Steegborn C, Sinclair D, Rolo A (2019) Mitohormesis and metabolic health: The interplay between ROS, cAMP and sirtuins. Free Radical Biol Med 141:483–491
Viña J, Gomez-Cabrera M, Borras C, Froio T, Sanchis-Gomar F, Martinez-Bello V, Pallardo F (2009) Mitochondrial biogenesis in exercise and in ageing. Adv Drug Deliv Rev 61(14):1369–1374
Naia L, Carmo C, Campesan S, Fão L, Cotton V, Valero J, Lopes C, Rosenstock T, Giorgini F, Rego A (2021) Mitochondrial SIRT3 confers neuroprotection in Huntington’s disease by regulation of oxidative challenges and mitochondrial dynamics. Free Radical Biol Med 163:163–179
Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D, Novelli R, Remuzzi G, Benigni A (2015) Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Investig 125(2):715–726
Y. Shen, Q. Wu, J. Shi, S. Zhou, Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson's disease, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 132 (2020) 110928.
Flynn J, Melov S (2013) SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radical Biol Med 62:4–12
Meng G, Liu J, Liu S, Song Q, Liu L, Xie L, Han Y, Ji Y (2018) Hydrogen sulfide pretreatment improves mitochondrial function in myocardial hypertrophy via a SIRT3-dependent manner. Br J Pharmacol 175(8):1126–1145
Tao R, Vassilopoulos A, Parisiadou L, Yan Y, Gius D (2014) Regulation of MnSOD enzymatic activity by Sirt3 connects the mitochondrial acetylome signaling networks to aging and carcinogenesis. Antioxid Redox Signal 20(10):1646–1654
Dikalova A, Itani H, Nazarewicz R, McMaster W, Flynn C, Uzhachenko R, Fessel J, Gamboa J, Harrison D, Dikalov S (2017) Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ Res 121(5):564–574
Zhong J, Tan Y, Lu J, Liu J, Xiao X, Zhu P, Chen S, Zheng S, Chen Y, Hu Y, Guo Z (2019) Therapeutic contribution of melatonin to the treatment of septic cardiomyopathy: A novel mechanism linking Ripk3-modified mitochondrial performance and endoplasmic reticulum function. Redox Biol 26:101287
H. Galley, B. McCormick, K. Wilson, D. Lowes, L. Colvin, C. Torsney, Melatonin limits paclitaxel-induced mitochondrial dysfunction in vitro and protects against paclitaxel-induced neuropathic pain in the rat, Journal of pineal research 63(4) (2017).
Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, García-Corzo L, López LC, Reiter RJ, Acuña-Castroviejo D (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res 52(2):217–227
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81770095, 81700093, 81901952, 8210082163), the Fundamental Research Funds for the Central Universities (No. 2042021kf0081) and Science Fund for Creative Research Groups of the Natural Science Foundation of Hubei Province (No. 2020CFA027).
Author information
Authors and Affiliations
Contributions
LN, XR and LG contributed equally to this work. LN, XR and LG designed, performed research, analyzed data and wrote the paper. FT, LD and XC performed experiments, analyzed data. WX and GQ helped to design experiments and reviewed the data. LN, WX and GQ helped to design the research, analyzed data and reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All authors consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ning, L., Rui, X., Guorui, L. et al. A novel mechanism for the protection against acute lung injury by melatonin: mitochondrial quality control of lung epithelial cells is preserved through SIRT3-dependent deacetylation of SOD2. Cell. Mol. Life Sci. 79, 610 (2022). https://doi.org/10.1007/s00018-022-04628-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04628-0