Abstract
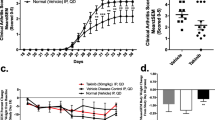
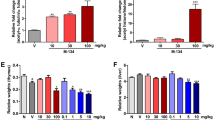
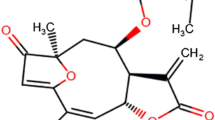
Hemarthrosis is the primary cause of hemophiliac arthropathy (HA). Pro-inflammatory cytokines are thought to play an important role in the pathogenesis of HA, and thus, anti-cytokine approaches may be used as an adjuvant therapy. A novel series of enaminone compounds (JODI), that contain the N-aryl piperazino motif, have been shown in vitro to reduce pro-inflammatory cytokines and thus may be efficacious in vivo. In this report, we will assess whether JODI can suppress multiple cytokines which might be potentially responsible for joint inflammation in a mouse model of hemarthrosis. The results showed that JODI significantly improved the survival after LPS treatment, and most pro-inflammatory cytokines/chemokines were decreased significantly after JODI administration. In the hemophilia mouse model, hemarthrosis resulted in local cytokine/chemokine changes, represented by elevated pro-inflammatory (IL-6, MCP-1, MIP-1α, MIP-1β) and pro-angiogenic (VEGF and IL-33) cytokines, and decreased anti-pro-inflammatory cytokines IL-4 and IL-10. The changes were reversed by administration of JODI, which can be used as a novel approach to manage hemophilia arthropathy.








Similar content being viewed by others
References
Srivastava, A., A.K. Brewer, E.P. Mauser-Bunschoten, et al. 2012. Guidelines for the management of hemophilia. Hemophilia 19: e1–e47.
Soucie, J.M., S.D. Grosse, A.E. Siddiqi, et al. 2017. The effects of joint disease, inhibitors and other complications on health-related quality of life among males with severe hemophilia A in the United States. Hemophilia 23: e287–e293.
Nilsson, I.M., E. Berntorp, T. Lofqvist, et al. 1992. Twenty-five years’ experience of prophylactic treatment in severe hemophilia A and B. Journal of Internal Medicine 232: 25–32.
Manco-Johnson, M.J., B. Lundin, S. Funk, C. Peterfy, D. Raunig, M. Werk, C.L. Kempton, M.T. Reding, S. Goranov, L. Gercheva, L. Rusen, V. Uscatescu, M. Pierdominici, S. Engelen, J. Pocoski, D. Walker, and W. Hong. 2017. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. Journal of Thrombosis and Haemostasis 15: 2115–2124.
Manco-Johnson, Marilyn J., Thomas C. Abshire, Amy D. Shapiro, Brenda Riske, Michele R. Hacker, Ray Kilcoyne, J. David Ingram, Michael L. Manco-Johnson, Sharon Funk, Linda Jacobson, Leonard A. Valentino, W. Keith Hoots, George R. Buchanan, Donna DiMichele, Michael Recht, Deborah Brown, Cindy Leissinger, Shirley Bleak, Alan Cohen, Prasad Mathew, Alison Matsunaga, Desiree Medeiros, Diane Nugent, Gregory A. Thomas, Alexis A. Thompson, Kevin McRedmond, J. Michael Soucie, Harlan Austin, and Bruce L. Evatt. 2007. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. The New England Journal of Medicine 357: 535–544.
Fischer, K., K. Steen Carlsson, P. Petrini, et al. 2013. Intermediate-dose versus high-dose prophylaxis for severe hemophilia: comparing outcome and costs since the 1970s. Blood 122: 1129–1136.
Simpson, M.L., and L.A. Valentino. 2012. Management of joint bleeding in hemophilia. Expert Review of Hematology 5: 459–468.
Arruda, V.R., B.S. Doshi, and B.J. Samelson-Jones. 2017. Novel approaches to hemophilia therapy: successes and challenges. Blood 130: 2251–2256.
Melchiorre, D., M. Manetti, and M. Matucci-Cerinic. 2017. Pathophysiology of hemophilic arthropathy. Journal of Clinical Medicine 6: 63.
Ovlisen, K., A.T. Kristensen, A.L. Jensen, et al. 2009. IL-1 beta, IL-6, KC and MCP-1 are elevated in synovial fluid from hemophilic mice with experimentally induced haemarthrosis. Hemophilia 15: 802–810.
Sen, D., A. Chapla, N. Walter, V. Daniel, A. Srivastava, and G.R. Jayandharan. 2013. Nuclear factor (NF)-kappaB and its associated pathways are major molecular regulators of blood-induced joint damage in a murine model of hemophilia. Journal of Thrombosis and Haemostasis 11: 293–306.
El-Hashim, A., S. Yousefi, I. Edafiogho, et al. 2010. Anti-inflammatory and immunosuppressive effects of the enaminone E121. European Journal of Pharmacology 632: 73–78.
Ghoneim, O.M., A. Bill, J. Dhuguru, D.E. Szollosi, and I.O. Edafiogho. 2018. Design, synthesis and biological evaluation of piperazino-enaminones as novel suppressants of pro-inflammatory cytokines. Bioorganic & Medicinal Chemistry 26: 3890–3898.
Szollosi, D.E., O.A. Ghoneim, M.K. Manzoor, et al. 2016. Novel piperazino-enaminones suppress pro-inflammatory cytokines and inhibit chemokine receptor CCR2. Inflammation 39: 2053–2061.
Lin, H.F., N. Maeda, O. Smithies, D.L. Straight, and D.W. Stafford. 1997. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood 90: 3962–3966.
Sun, J., N. Hakobyan, L.A. Valentino, B.L. Feldman, R.J. Samulski, and P.E. Monahan. 2008. Intraarticular factor IX protein or gene replacement protects against development of hemophilic synovitis in the absence of circulating factor IX. Blood 112: 4532–4541.
Sun, J., B. Hua, E.W. Livingston, S. Taves, P.B. Johansen, M. Hoffman, M. Ezban, D.M. Monroe, T.A. Bateman, and P.E. Monahan. 2017. Abnormal joint and bone wound healing in hemophilia mice is improved by extending factor IX activity after hemarthrosis. Blood 129: 2161–2171.
Valentino, L.A., N. Hakobyan, C. Enockson, et al. 2012. Exploring the biological basis of hemophilic joint disease: experimental studies. Hemophilia 18: 310–318.
Drake, T.A., J.H. Morrissey, and T.S. Edgington. 1989. Selective cellular expression of tissue factor in human tissues, implications for disorders of hemostasis and thrombosis. The American Journal of Pathology 134: 1087–1097.
Storti, E., U. Magrini, and E. Ascari. 1971. Synovial fibrinolysis and hemophilic hemarthrosis. British Medical Journal 4: 812.
Deschaseaux, F., L. Sensebe, and D. Heymann. 2009. Mechanisms of bone repair and regeneration. Trends in Molecular Medicine 15: 417–429.
Baud'huin, M., F. Lamoureux, L. Duplomb, et al. 2007. RANKL, RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases. Cellular and Molecular Life Sciences 64: 2334–2350.
Kaneshiro, S., K. Ebina, K. Shi, C. Higuchi, M. Hirao, M. Okamoto, K. Koizumi, T. Morimoto, H. Yoshikawa, and J. Hashimoto. 2014. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. Journal of Bone and Mineral Metabolism 32: 378–392.
Sims, N.A., B.J. Jenkins, J.M. Quinn, et al. 2004. Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. The Journal of Clinical Investigation 113: 379–389.
van Vulpen, L.F., R.E. Schutgens, K. Coeleveld, et al. 2015. IL-1beta, in contrast to TNF alpha, is pivotal in blood-induced cartilage damage and is a potential target for therapy. Blood 126: 2239–2246.
Cavalli, G., and C.A. Dinarello. 2015. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology 54: 2134–2144.
Latourte, A., A. Frazier, C. Briere, et al. 2013. Interleukin-1 receptor antagonist in refractory haemochromatosis-related arthritis of the hands. Annals of the Rheumatic Diseases 72: 783–784.
Narkbunnam, N., J. Sun, G. Hu, F.C. Lin, T.A. Bateman, M. Mihara, and P.E. Monahan. 2013. IL-6 receptor antagonist as adjunctive therapy with clotting factor replacement to protect against bleeding-induced arthropathy in hemophilia. Journal of Thrombosis and Haemostasis 11: 881–893.
Sun, Junjiang, Genlin Hu, and Paul E. Monahan. 2009. TNF-alpha antagonists augment factor replacement to prevent arthropathy in hemophilic mice. Journal of Thrombosis and Haemostasis 7 (Suppl 2): 225.
Melchiorre, D., M. Morfini, S. Linari, A.L. Zignego, M. Innocenti, and M. Matucci Cerinic. 2014. Anti-TNF-alpha therapy prevents the recurrence of joint bleeding in hemophilia and arthritis. Rheumatology 53: 576–578.
McDonald, A.G., K. Yang, H.R. Roberts, D.M. Monroe, and M. Hoffman. 2008. Perivascular tissue factor is down-regulated following cutaneous wounding: implications for bleeding in hemophilia. Blood 111: 2046–2048.
Acharya, S.S., R.N. Kaplan, D. Macdonald, O.T. Fabiyi, D. DiMichele, and D. Lyden. 2011. Neoangiogenesis contributes to the development of hemophilic synovitis. Blood 117: 2484–2493.
He, R., H. Yin, B. Yuan, T. Liu, L. Luo, P. Huang, L. Dai, and K. Zeng. 2017. IL-33 improves wound healing through enhanced M2 macrophage polarization in diabetic mice. Molecular Immunology 90: 42–49.
Nefla, M., D. Holzinger, F. Berenbaum, and C. Jacques. 2016. The danger from within: alarmins in arthritis. Nature Reviews Rheumatology 12: 669–683.
Nieuwenhuizen, L., R.E. Schutgens, K. Coeleveld, et al. 2014. Hemarthrosis in hemophilic mice results in alterations in M1-M2 monocyte/macrophage polarization. Thrombosis Research 133: 390–395.
van Meegeren, M.E., G. Roosendaal, N.W. Jansen, et al. 2012. IL-4 alone and in combination with IL-10 protects against blood-induced cartilage damage. Osteoarthritis and Cartilage 20: 764–772.
van Meegeren, M.E., G. Roosendaal, K. van Veghel, et al. 2013. A short time window to profit from protection of blood-induced cartilage damage by IL-4 plus IL-10. Rheumatology 52: 1563–1571.
van Meegeren, M.E., G. Roosendaal, K. Coeleveld, et al. 2013. A single intra-articular injection with IL-4 plus IL-10 ameliorates blood-induced cartilage degeneration in hemophilic mice. British J Hematology 160: 515–520.
Acknowledgments
The authors acknowledge the Animal Histopathology and Lab Medicine Core at UNC-CH for multiple cytokine measurements and hematological and liver/kidney tests.
Funding
This work was partly supported by a research grant from Asklepios BioPharmaceutical (to J.S.). It was also supported by “the Fundamental Research Funds for the Central Universities.”
Author information
Authors and Affiliations
Contributions
All authors reviewed the article and approved its content. JS and DS designed the study. CZ and JS performed the in vivo study and wrote the manuscript. DS, OG, AB, and IE prepared the compound and revised the manuscript. BH and YZ revised the manuscript.
Corresponding authors
Ethics declarations
All investigations were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOC 67 kb)
Rights and permissions
About this article
Cite this article
Zhong, C., Szollosi, D., Sun, J. et al. Novel Piperazino-Enaminones Decrease Pro-inflammatory Cytokines Following Hemarthrosis in a Hemophilia Mouse Model. Inflammation 42, 1719–1729 (2019). https://doi.org/10.1007/s10753-019-01032-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01032-y