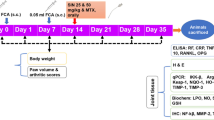
Abstract
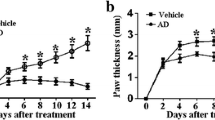
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by debilitating pain, cartilage destruction, and loss of joint function. Management of RA includes drugs that target NF-κB and downstream cytokine production. Therefore, molecules that act by inhibiting this signaling pathway without the severe side effects of, for instance, corticoids would be suitable therapeutic strategies. Budlein A is a sesquiterpene lactone with antinociceptive and anti-inflammatory properties related to the inhibition of pro-inflammatory cytokines and neutrophil recruitment. In this study, the effect of budlein A was evaluated in antigen-induced arthritis (AIA) in mice. At the 26th day, leukocyte recruitment to the knee joint, knee contents of proteoglycans, blood levels of ALT and AST, stomach tissue myeloperoxidase activity, and RT-qPCR for pro-inflammatory gene mRNA expression in knee joint samples was performed. NF-κB luciferase activity was evaluated in RAW 264.7 macrophages. Budlein A treatment dose-dependently inhibited AIA-induced mechanical hyperalgesia, edema, total leukocytes and neutrophil recruitment, and proteoglycan degradation. Budlein A did not induce gastric or liver damage. Budlein also inhibited AIA-induced Il-33, Tnf, Il-1β, preproET-1, and Cox-2 mRNA expression. In vitro, budlein reduced TNF- and IL-1β-induced NF-κB activity in RAW 264.7 macrophages. Altogether, we demonstrate that budlein A ameliorates AIA-induced inflammation and pain by targeting NF-κB. Importantly, budlein A does not induce in vivo side effects, suggesting that it possesses a favorable pre-clinical profile as analgesic and it is a prosperous molecule to be further investigated for the treatment of RA.








Similar content being viewed by others
Change history
06 September 2017
An erratum to this article has been published.
References
McInnes, I.B., and G. Schett. 2007. Cytokines in the pathogenesis of rheumatoid arthritis. Nature Reviews. Immunology 7: 429–442.
Burmester, G.R., E. Feist, and T. Dorner. 2014. Emerging cell and cytokine targets in rheumatoid arthritis. Nature Reviews. Rheumatology 10: 77–88.
Fattori, V., F.A. Amaral, and W.A. Verri Jr. 2016. Neutrophils and arthritis: Role in disease and pharmacological perspectives. Pharmacological Research 112: 84–98.
Ferreira, J.F., A.A. Ahmed Mohamed, and P. Emery. 2016. Glucocorticoids and rheumatoid arthritis. Rheumatic Diseases Clinics of North America 42: 33–46 vii.
M.S.N. Hohmann, D.T. Longhi-Balbinot, C.F.S. Guazelli, S.A. Navarro, A.C. Zarpelon, R. Casagrande, N.S. Arakawa, W.A. Verri Jr. 2016. Chapter 7 - Sesquiterpene Lactones: Structural Diversity and Perspectives as Anti-Inflammatory Molecules, in: R. Atta ur (Ed.) Studies in Natural Products Chemistry, Elsevier, pp. 243–264.
Gao, S., Q. Wang, X.H. Tian, H.L. Li, Y.H. Shen, X.K. Xu, G.Z. Wu, Z.L. Hu, and W.D. Zhang. 2017. Total sesquiterpene lactones prepared from Inula Helenium L. has potentials in prevention and therapy of rheumatoid arthritis. Journal of Ethnopharmacology 196: 39–46.
Valerio, D.A., T.M. Cunha, N.S. Arakawa, H.P. Lemos, F.B. Da Costa, C.A. Parada, S.H. Ferreira, F.Q. Cunha, and W.A. Verri Jr. 2007. Anti-inflammatory and analgesic effects of the sesquiterpene lactone budlein a in mice: Inhibition of cytokine production-dependent mechanism. European Journal of Pharmacology 562: 155–163.
Nicolete, R., N.S. Arakawa, C. Rius, A. Nomizo, P.J. Jose, F.B. Da Costa, M.J. Sanz, and L.H. Faccioli. 2009. Budlein a from Viguiera Robusta inhibits leukocyte-endothelial cell interactions, adhesion molecule expression and inflammatory mediators release. Phytomedicine 16: 904–915.
Knob, C.D., M. Silva, T.H. Gasparoto, C.E. Oliveira, N.G. Amor, N.S. Arakawa, F.B. Costa, and A.P. Campanelli. 2016. Effects of budlein a on human neutrophils and lymphocytes. Journal of Applied Oral Science 24: 271–277.
Arakawa, N.S., K. Schorr, S.R. Ambrosio, I. Merfort, and F.B. Da Costa. 2008. Further sesquiterpene lactones from Viguiera Robusta and the potential anti-inflammatory activity of a heliangolide: Inhibition of human neutrophil elastase release. Zeitschrift für Naturforschung. Section C 63: 533–538.
Lyss, G., A. Knorre, T.J. Schmidt, H.L. Pahl, and I. Merfort. 1998. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. The Journal of Biological Chemistry 273: 33508–33516.
Widen, J.C., A.M. Kempema, P.W. Villalta, and D.A. Harki. 2017. Targeting NF-kappaB p65 with a Helenalin inspired Bis-electrophile. ACS Chemical Biology 12: 102–113.
Zhao, Y., S.J. Chen, J.C. Wang, H.X. Niu, Q.Q. Jia, X.W. Chen, X.Y. Du, L. Lu, B. Huang, Q. Zhang, Y. Chen, and H.B. Long. 2015. Sesquiterpene lactones inhibit advanced oxidation protein product-induced MCP-1 expression in podocytes via an IKK/NF-kappaB-dependent mechanism. Oxidative Medicine and Cellular Longevity 2015: 934058.
Simmonds, R.E., and B.M. Foxwell. 2008. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology (Oxford) 47: 584–590.
Da Costa F.B., Schorr K., Arakawa N.S., Schilling E.E., Spring O. 2001. Infraspecific Variation in the Chemistry of Glandular Trichomes of two Brazilian Viguiera Species (Heliantheae; Asteraceae). Journal of the Brazilian Chemical Society 12: 403–407
Verri, W.A., Jr., A.T. Guerrero, S.Y. Fukada, D.A. Valerio, T.M. Cunha, D. Xu, S.H. Ferreira, F.Y. Liew, and F.Q. Cunha. 2008. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proceedings of the National Academy of Sciences of the United States of America 105: 2723–2728.
Guerrero, A.T., W.A. Verri Jr., T.M. Cunha, T.A. Silva, F.A. Rocha, S.H. Ferreira, F.Q. Cunha, and C.A. Parada. 2006. Hypernociception elicited by tibio-tarsal joint flexion in mice: A novel experimental arthritis model for pharmacological screening. Pharmacology, Biochemistry, and Behavior 84: 244–251.
Ruiz-Miyazawa K.W., Staurengo-Ferrari L., Mizokami S.S., Domiciano T.P., Vicentini F., Camilios-Neto D., Pavanelli W.R., Pinge-Filho P., Amaral F.A., Teixeira M.M., Casagrande R., Verri W.A., Jr. 2017. Quercetin inhibits gout arthritis in mice: induction of an opioid-dependent regulation of inflammasome. Inflammopharmacology 1–16. doi:10.1007/s10787-017-0356-x.
Verri, W.A., Jr., F.O. Souto, S.M. Vieira, S.C. Almeida, S.Y. Fukada, D. Xu, J.C. Alves-Filho, T.M. Cunha, A.T. Guerrero, R.B. Mattos-Guimaraes, F.R. Oliveira, M.M. Teixeira, J.S. Silva, I.B. McInnes, S.H. Ferreira, P. Louzada-Junior, F.Y. Liew, and F.Q. Cunha. 2010. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Annals of the Rheumatic Diseases 69: 1697–1703.
Casagrande, R., S.R. Georgetti, W.A. Verri Jr., D.J. Dorta, A.C. dos Santos, and M.J. Fonseca. 2006. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. Journal of Photochemistry and Photobiology. B 84: 21–27.
Pinto, L.G., T.M. Cunha, S.M. Vieira, H.P. Lemos, W.A. Verri Jr., F.Q. Cunha, and S.H. Ferreira. 2010. IL-17 mediates articular hypernociception in antigen-induced arthritis in mice. Pain 148: 247–256.
Verri, W.A., Jr., T.M. Cunha, C.A. Parada, X.Q. Wei, S.H. Ferreira, F.Y. Liew, and F.Q. Cunha. 2006. IL-15 mediates immune inflammatory hypernociception by triggering a sequential release of IFN-gamma, endothelin, and prostaglandin. Proceedings of the National Academy of Sciences of the United States of America 103: 9721–9725.
Talbot, J., F.J. Bianchini, D.C. Nascimento, R.D. Oliveira, F.O. Souto, L.G. Pinto, R.S. Peres, J.R. Silva, S.C. Almeida, P. Louzada-Junior, T.M. Cunha, F.Q. Cunha, and J.C. Alves-Filho. 2015. CCR2 expression in neutrophils plays a critical role in their migration into the joints in rheumatoid arthritis. Arthritis & Rhematology 67: 1751–1759.
Cunha, T.M., W.A. Verri Jr., I.R. Schivo, M.H. Napimoga, C.A. Parada, S. Poole, M.M. Teixeira, S.H. Ferreira, and F.Q. Cunha. 2008. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. Journal of Leukocyte Biology 83: 824–832.
Guerrero, A.T., W.A. Verri Jr., T.M. Cunha, T.A. Silva, I.R. Schivo, D. Dal-Secco, C. Canetti, F.A. Rocha, C.A. Parada, F.Q. Cunha, and S.H. Ferreira. 2008. Involvement of LTB4 in zymosan-induced joint nociception in mice: Participation of neutrophils and PGE2. Journal of Leukocyte Biology 83: 122–130.
Sachs, D., F.Q. Cunha, and S.H. Ferreira. 2004. Peripheral analgesic blockade of hypernociception: Activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proceedings of the National Academy of Sciences of the United States of America 101: 3680–3685.
Cunha, T.M., D. Roman-Campos, C.M. Lotufo, H.L. Duarte, G.R. Souza, W.A. Verri Jr., M.I. Funez, Q.M. Dias, I.R. Schivo, A.C. Domingues, D. Sachs, S. Chiavegatto, M.M. Teixeira, J.S. Hothersall, J.S. Cruz, F.Q. Cunha, and S.H. Ferreira. 2010. Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proceedings of the National Academy of Sciences of the United States of America 107: 4442–4447.
Jin, X., and R.W.T. Gereau. 2006. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. The Journal of Neuroscience 26: 246–255.
Binshtok, A.M., H. Wang, K. Zimmermann, F. Amaya, D. Vardeh, L. Shi, G.J. Brenner, R.R. Ji, B.P. Bean, C.J. Woolf, and T.A. Samad. 2008. Nociceptors are interleukin-1beta sensors. The Journal of Neuroscience 28: 14062–14073.
Gokin, A.P., M.U. Fareed, H.L. Pan, G. Hans, G.R. Strichartz, and G. Davar. 2001. Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. The Journal of Neuroscience 21: 5358–5366.
Kolaczkowska, E., and P. Kubes. 2013. Neutrophil recruitment and function in health and inflammation. Nature Reviews. Immunology 13: 159–175.
Zarpelon, A.C., F.C. Rodrigues, A.H. Lopes, G.R. Souza, T.T. Carvalho, L.G. Pinto, D. Xu, S.H. Ferreira, J.C. Alves-Filho, I.B. McInnes, B. Ryffel, V.F. Quesniaux, F. Reverchon, S. Mortaud, A. Menuet, F.Y. Liew, F.Q. Cunha, T.M. Cunha, and W.A. Verri Jr. 2016. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. The FASEB Journal 30: 54–65.
Zarpelon, A.C., T.M. Cunha, J.C. Alves-Filho, L.G. Pinto, S.H. Ferreira, I.B. McInnes, D. Xu, F.Y. Liew, F.Q. Cunha, and W.A. Verri Jr. 2013. IL-33/ST2 signalling contributes to carrageenin-induced innate inflammation and inflammatory pain: Role of cytokines, endothelin-1 and prostaglandin E2. British Journal of Pharmacology 169: 90–101.
Siedle, B., A.J. Garcia-Pineres, R. Murillo, J. Schulte-Monting, V. Castro, P. Rungeler, C.A. Klaas, F.B. Da Costa, W. Kisiel, and I. Merfort. 2004. Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-kappaB. Journal of Medicinal Chemistry 47: 6042–6054.
Garcia-Pineres, A.J., V. Castro, G. Mora, T.J. Schmidt, E. Strunck, H.L. Pahl, and I. Merfort. 2001. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. The Journal of Biological Chemistry 276: 39713–39720.
Acknowledgment and Funding
This work was supported by grants from Departamentode Ciência e Tecnologia da Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Ministério da Saúde (Decit/SCTIE/MS, Brazil) intermediated by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) with support of Fundação Araucária and Secretaria Estadual de Saúde, Paraná (SESA-PR, Brazil); São Paulo Research Foundation under grant agreements 2011/19670-0 (Thematic Project) and 2013/08216-2 (Center for Research in Inflammatory Disease); Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil); and Financiadora de Estudos e Projetos and Secretaria de Estado da Ciência, Tecnologia e Ensino Superior do Paraná under grant agreements 01.12.0294.00 (0476/11) (FINEP/SETI-PR, Brazil). ACZ received CAPES/Fundação Araucária Post-Doc fellowship. TMC, FBC, FQC, RC, and WAVJ receive senior research fellowship from CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were conducted in accordance with animal care and handling procedures of the International Association for Study of Pain (IASP) and with the approval Londrina State University Ethics Committee on Animal Research and Welfare (protocol number 14544.2013.46).
Competing Interests
The authors declare that they have no competing interests.
Additional information
An erratum to this article is available at https://doi.org/10.1007/s10753-017-0663-9.
Rights and permissions
About this article
Cite this article
Zarpelon, A.C., Fattori, V., Souto, F.O. et al. The Sesquiterpene Lactone, Budlein A, Inhibits Antigen-Induced Arthritis in Mice: Role of NF-κB and Cytokines. Inflammation 40, 2020–2032 (2017). https://doi.org/10.1007/s10753-017-0642-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0642-1