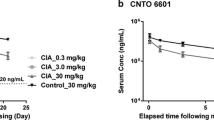
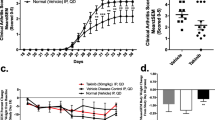
Abstract
Background
Although tofacitinib has shown highly significant efficacy for rheumatoid arthritis (RA), there are still a considerable number of patients that are non-responders owing to its limited effectiveness and various adverse effects. Thus, alternative options with better efficacy and lower toxicity are desired. Here, M-134, a recently developed HDAC6 inhibitor, was examined for its therapeutic potential when combined with tofacitinib in a rat model of RA.
Methods
The single or combined administration of M-134 and tofacitinib was examined in complete Freund’s adjuvant-induced arthritis (AIA) or collagen-induced arthritis (CIA) rodent models. To evaluate the therapeutic and adverse effects, the following factors were observed: macroscopic or microscopic scoring of all four paws; the expression of ICAM-1, VCAM-1, and IP-10 in the joints and that of various cytokines and chemokines in the plasma; the weight of the thymus and the liver; and changes in hematological enzymes.
Results
Combination treatment showed strong synergistic effects as measured by the clinical score and histological changes, without adverse effects such as weight loss in the thymus and increased liver enzymes (ALT and AST). Additionally, it also reduced ICAM-1, VCAM-1, and IP-10 expression in the joints, and M-134 increased the efficacy of tofacitinib by regulating various cytokines, such as interleukin (IL)-1β, IL-17, and TNF-α, in the serum of AIA rats. Differences in the cytokine expression for each drug were found in the CIA model.
Conclusions
M-134 and tofacitinib combination therapy is a potential option for the treatment of RA through the regulation of cytokines, chemokines, and adhesion molecules.







Similar content being viewed by others
Abbreviations
- AIA:
-
Adjuvant-induced arthritis
- CIA:
-
Collagen-induced arthritis
- HDAC:
-
Histone deacetylase
- IL:
-
Interleukin
- JAK:
-
Janus kinase
- RA:
-
Rheumatoid arthritis
- TNF:
-
Tumor necrosis factor
- TGF:
-
Transforming growth factor
References
Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–93.
Quan LD, Thiele GM, Tian J, Wang D. The development of novel therapies for rheumatoid arthritis. Expert Opin Ther Pat. 2008;18:723–38.
Llop-Guevara A, Porras M, Cendon C, Di Ceglie I, Siracusa F, Madarena F, et al. Simultaneous inhibition of JAK and SYK kinases ameliorates chronic and destructive arthritis in mice. Arthritis Res Ther. 2015;17:356.
Hull EE, Montgomery MR, Leyva KJ. HDAC Inhibitors as epigenetic regulators of the immune system: impacts on cancer therapy and inflammatory diseases. Biomed Res Int. 2016:8797206.
Lin HS, Hu CY, Chan HY, Liew YY, Huang HP, Lepescheux L, et al. Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. Br J Pharmacol. 2007;150:862–72.
Glauben R, Batra A, Fedke I, Zeitz M, Lehr HA, Leoni F, et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol. 2006;176:5015–22.
Joosten LA, Leoni F, Meghji S, Mascagni P. Inhibition of HDAC activity by ITF2357 ameliorates joint inflammation and prevents cartilage and bone destruction in experimental arthritis. Mol Med. 2011;17:391–6.
Subramanian S, Bates SE, Wright JJ, Espinoza-Delgado I, Piekarz RL. Clinical toxicities of histone deacetylase inhibitors. Pharmaceuticals (Basel). 2010;3:2751–67.
Lee JW, Lee SM, Chun J, Im JP, Seo SK, Ha N, et al. Novel histone deacetylase 6 inhibitor M-134 inhibits NF-kappaB signaling in intestinal epithelial cells and macrophages and ameliorates acute and chronic murine colitis. Inflamm Bowel Dis. 2020;26:852–62.
Li Y, Shin D, Kwon SH. Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes. FEBS J. 2013;280:775–93.
Lee JH, Mahendran A, Yao Y, Ngo L, Venta-Perez G, Choy ML, et al. Development of a histone deacetylase 6 inhibitor and its biological effects. Proc Natl AcadSci U S A. 2013;110:15704–9.
de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, et al. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol Cell Biol. 2011;31:2066–78.
Into T, Inomata M, Niida S, Murakami Y, Shibata K. Regulation of MyD88 aggregation and the MyD88-dependent signaling pathway by sequestosome 1 and histone deacetylase 6. J Biol Chem. 2010;285:35759–69.
Serrador JM, Cabrero JR, Sancho D, Mittelbrunn M, Urzainqui A, Sanchez-Madrid F. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity. 2004;20:417–28.
Youn GS, Lee KW, Choi SY, Park J. Overexpression of HDAC6 induces pro-inflammatory responses by regulating ROS-MAPK-NF-kappaB/AP-1 signaling pathways in macrophages. Free Radic Biol Med. 2016;97:14–23.
Choi EW, Song JW, Ha N, Choi YI, Kim S. M-134, a novel HDAC6-selective inhibitor, improves renal outcomes and survival in a mouse model of systemic lupus erythematosus. Sci Rep. 2018;8:17297.
Oh BR, Suh DH, Bae D, Ha N, Choi YI, Yoo HJ, et al. Therapeutic effect of a novel histone deacetylase 6 inhibitor, CKD-L, on collagen-induced arthritis in vivo and regulatory T cells in rheumatoid arthritis in vitro. Arthritis Res Ther. 2017;19:154.
Zhang Y, Kwon S, Yamaguchi T, Cubizolles F, Rousseaux S, Kneissel M, et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28:1688–701.
O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–28.
Malemud CJ. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2018;10:117–27.
Cutolo M. The kinase inhibitor tofacitinib in patients with rheumatoid arthritis: latest findings and clinical potential. Ther Adv Musculoskelet Dis. 2013;5:3–11.
Virtanen AT, Haikarainen T, Raivola J, Silvennoinen O. Selective JAKinibs: prospects in inflammatory and autoimmune diseases. Bio Drugs. 2019;33:15–32.
Wollenhaupt J, Lee EB, Curtis JR, Silverfield J, Terry K, Soma K, et al. Safety and efficacy of tofacitinib for up to 95 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21:89.
Kivitz AJ, Cohen S, Keystone E, van Vollenhoven RF, Haraoui B, Kaine J, et al. A pooled analysis of the safety of tofacitinib as monotherapy or in combination with background conventional synthetic disease-modifying antirheumatic drugs in a Phase 3 rheumatoid arthritis population. Semin Arthritis Rheum. 2018;48:406–15.
Whiteley PE, Dalrymple SA. Models of inflammation: adjuvant-induced arthritis in the rat. Curr Protoc Pharmacol. 2001;Chapter 5:Unit5 5.
Inglis JJ, Simelyte E, McCann FE, Criado G, Williams RO. Protocol for the induction of arthritis in C57BL/6 mice. Nat Protoc. 2008;3:612–8.
He Y, Wong AY, Chan EW, Lau WC, Man KK, Chui CS, et al. Efficacy and safety of tofacitinib in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2013;14:298.
Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol. 2008;84:915–23.
Clinical Pharmacology Review. NDA203214. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203214Orig1s000PharmR.pdf.
Kotyla PJ. Are Janus kinase inhibitors superior over classic biologic agents in RA patients? Biomed Res Int. 2018:7492904.
Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322.
Dowty ME, Lin TH, Jesson MI, Hegen M, Martin DA, Katkade V, et al. Janus kinase inhibitors for the treatment of rheumatoid arthritis demonstrate similar profiles of in vitro cytokine receptor inhibition. Pharmacol Res Perspect. 2019;7:e00537.
Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–87.
Farrugia M, Baron B. The role of TNF-alpha in rheumatoid arthritis: a focus on regulatory T cells. J Clin Transl Res. 2016;2:84–90.
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15.
Eastgate JA, Symons JA, Wood NC, Grinlinton FM, di Giovine FS, Duff GW. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988;2:706–9.
Arend WP. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J Clin Invest. 1991;88:1445–51.
Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365–70.
Gonzalo-Gil E, Criado G, Santiago B, Dotor J, Pablos JL, Galindo M. Transforming growth factor (TGF)-beta signalling is increased in rheumatoid synovium but TGF-beta blockade does not modify experimental arthritis. Clin Exp Immunol. 2013;174:245–55.
Bira Y, Tani K, Nishioka Y, Miyata J, Sato K, Hayashi A, et al. Transforming growth factor beta stimulates rheumatoid synovial fibroblasts via the type II receptor. Mod Rheumatol. 2005;15:108–13.
Ahmed S, Riegsecker S, Beamer M, Rahman A, Bellini JV, Bhansali P, et al. Largazole, a class I histone deacetylase inhibitor, enhances TNF-alpha-induced ICAM-1 and VCAM-1 expression in rheumatoid arthritis synovial fibroblasts. Toxicol Appl Pharmacol. 2013;270:87–96.
Wang L, Ding Y, Guo X, Zhao Q. Role and mechanism of vascular cell adhesion molecule-1 in the development of rheumatoid arthritis. Exp Ther Med. 2015;10:1229–33.
van Hooij A, Boeters DM, TjonKon Fat EM, van den Eeden SJF, Corstjens P, van der Helm-van Mil AHM, et al. Longitudinal IP-10 serum levels are associated with the course of disease activity and remission in patients with rheumatoid arthritis. Clin Vaccine Immunol. 2017;24:e00060-e117.
Howard Tripp N, Tarn J, Natasari A, Gillespie C, Mitchell S, Hackett KL, et al. Fatigue in primary Sjogren’s syndrome is associated with lower levels of proinflammatory cytokines. RMD Open. 2016;2:e000282.
Fischer BD, Adeyemo A, O’Leary ME, Bottaro A. Animal models of rheumatoid pain: experimental systems and insights. Arthritis Res Ther. 2017;19:146.
Hegen M, Keith JC Jr, Collins M, Nickerson-Nutter CL. Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann Rheum Dis. 2008;67:1505–15.
Gao B, Lin J, Jiang Z, Yang Z, Yu H, Ding L, et al. Upregulation of chemokine CXCL10 enhances chronic pulmonary inflammation in tree shrew collagen-induced arthritis. Sci Rep. 2018;8:9993.
Funding
We would like to thank the scientists of the Department of Pharmacology, Chong Kun Dang Hyo-Jong Research Institute for their technical support and assistance. This study was supported by research grants from CKD Pharmaceuticals.
Author information
Authors and Affiliations
Contributions
WCS and DKB were responsible for the study design. DKB, DHS, JTB, JSP, and JYL performed the animal test and histopathological experiments. DKB performed histological and molecular biological analyses. DKB, NH, YIC, and WCS interpreted the results, contributed to the discussion, and edited the manuscript. All the authors contributed to the interpretation of data and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
DKB, YIC, NH, DHS, and JYB are employees of CKD Research Institute. The other authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bae, D., Choi, Y., Lee, J. et al. M-134, a novel HDAC6-selective inhibitor, markedly improved arthritic severity in a rodent model of rheumatoid arthritis when combined with tofacitinib. Pharmacol. Rep 73, 185–201 (2021). https://doi.org/10.1007/s43440-020-00188-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00188-x